Abstract
Purpose
Increasing intracellular energy storage by chemically activating adenosine monophosphate–activated protein kinase (AMPKα) prior to sperm cryopreservation may improve post-thawed sperm function. Using the domestic cat as a biomedical model, the objectives were to (1) confirm the expression of AMPKα and its regulatory kinases in epididymal spermatozoa and (2) assess the influence of AMPK activator, 5′-aminoimidasole-4-carboxamide-1-β-d-ribofuranoside (AICAR) on epididymal sperm function before and after cryopreservation.
Methods
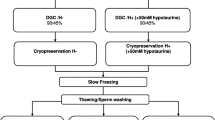
In study I, sperm samples of different qualities were obtained from cauda epididymides of domestic cats and evaluated for AMPKα expression. In study II, epididymal spermatozoa were equilibrated for either 30 or 60 min in the presence of 0 (control), 0.5, 2.0, and 5.0 mM AICAR and sperm functions were assessed before and after cryopreservation. In study III, epididymal spermatozoa were treated as in study II and evaluated for AMPKα signaling protein expressions (phospho-AMPKα Thr172 and GLUT1) as well as ATP levels.
Results
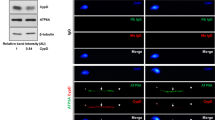
AMPKα protein expression was higher in high-motility vs poor-motility samples. Thirty-minute equilibration with 0.5 mM AICAR improved motion characteristics and fertilizing ability of cryopreserved sperm to the control. Increased expressions of phospho-AMPKα Thr172 and GLUT1 as well as intracellular ATP level were confirmed in sperm samples equilibrated with 0.5 or 2.0 mM AICAR for 30 min.
Conclusions
Presence and role of AMPKα protein in cat regulating sperm function were demonstrated before and after cryopreservation. Findings could be used to potentially enhance cryopreserved sperm function in sub-fertile men.



Similar content being viewed by others
References
Van Blerkom J. Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction. 2004;128:269–80.
Ramalho-Santos J, Varum S, Amaral S, Mota PC, Sousa AP, Amaral A. Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Update. 2009;15:553–72.
Ruiz-Pesini E, Díez-Sánchez C, López-Pérez MJ, Enríquez JA. The role of the mitochondrion in sperm function: is there a place for oxidative phosphorylation or is this a purely glycolytic process?(Review). Curr Top Dev Biol. 2007;77:3–19.
Peña FJ, Rodríguez Martínez H, Tapia JA, Ortega Ferrusola C, González Fernández L, Macías García B. Mitochondria in mammalian sperm physiology and pathology: a review. Reprod Domest Anim. 2009;44(2):345–9.
Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895–908.
Gaidhu MP, Fediuc S, Ceddia RB. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside-induced AMP-activated protein kinase phosphorylation inhibits basal and insulin-stimulated glucose uptake, lipid synthesis, and fatty acid oxidation in isolated rat adipocytes. J Biol Chem. 2006;281(36):25956–64.
Gaidhu MP, Fediuc S, Anthony NM, So M, Mirpourian M, Perry RL, et al. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J Lipid Res. 2009;50(4):704–15.
Bertoldo MJ, Guibert E, Faure M, Ramé C, Foretz M, Viollet B, et al. Specific deletion of AMP-activated protein kinase (α1AMPK) in murine oocytes alters junctional protein expression and mitochondrial physiology. PLoS One. 2015;10(3):e0119680.
Tartarin P, Guibert E, Touré A, Ouiste C, Leclerc J, Sanz N, et al. Inactivation of AMPKα1 induces asthenozoospermia and alters spermatozoa morphology. Endocrinology. 2012;153(7):3468–81.
Hurtado de Llera A, Martin-Hidalgo D, Gil MC, Garcia-Marin LJ, Bragado MJ. AMP-activated kinase AMPK is expressed in boar spermatozoa and regulates motility. PLoS One. 2012;7(6):e38840.
Nguyen TM, Seigneurin F, Froment P, Combarnous Y, Blesbois E. The 5'-AMP-activated protein kinase (AMPK) is involved in the augmentation of antioxidant defenses in cryopreserved chicken sperm. PLoS One. 2015;10(7):e0134420.
Calle-Guisado V, de Llera AH, Martin-Hidalgo D, Mijares J, Gil MC, Alvarez IS, et al. AMP-activated kinase in human spermatozoa: identification, intracellular localization, and key function in the regulation of sperm motility. Asian J Androl. 2017;19(6):707–14.
Shabani Nashtaei M, Nekoonam S, Naji M, Bakhshalizadeh S, Amidi F. Cryoprotective effect of resveratrol on DNA damage and crucial human sperm messenger RNAs, possibly through 5′ AMP-activated protein kinase activation. Cell Tissue Bank. 2017;26:87–95. https://doi.org/10.1007/s10561-017-9642-5.
Comizzoli P, Paulson EE, McGinnis L. The mutual benefits of research in wild animal species and human-assisted reproduction. J Assist Reprod Genet. 2018;35(4):551–60.
Thuwanut P, Chatdarong K, Johannisson A, Bergqvist AS, Söderquist L, Axnér E. Cryopreservation of epididymal cat spermatozoa: effects of in vitro antioxidative enzymes supplementation and lipid peroxidation induction. Theriogenology. 2010;73(8):1076–87.
Axnér E, Hermansson U, Linde-Forsberg C. The effect of Equex STM paste and sperm morphology on post-thaw survival of cat epididymal spermatozoa. Anim Reprod Sci. 2004;84:179–91.
Rota A, Ström B, Linde-Forsberg C, Rodriguez-Martinez H. Effects of Equex STM paste on viability of frozen-thawed dog spermatozoa during in vitro incubation at 38 degrees C. Theriogenology. 1997;47:1093–101.
Thuwanut P, Arya N, Comizzoli P, Chatdarong K. Effect of extracellular adenosine 5′-triphosphate on cryopreserved epididymal cat sperm intracellular ATP concentration, sperm quality, and in vitro fertilizing ability. Theriogenology. 2015;84(5):702–9.
Thuwanut P, Chatdarong K, Bergqvist AS, Söderquist L, Thiangtum K, Tongthainan D, et al. The effects of antioxidants on semen traits and in vitro fertilizing ability of sperm from the flat-headed cat (Prionailurus planiceps). Theriogenology. 2011;76(1):115–25.
Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J. 2000;346(Pt 3):659–69.
Martin-Hidalgo D, Hurtado de Llera A, Yeste M, Cruz Gil M, Bragado MJ, Garcia-Marin LJ. Adenosine monophosphate-activated kinase, AMPK, is involved in the maintenance of the quality of extended boar semen during long-term storage. Theriogenology. 2013;80(4):285–94.
Amaral A, Lourenço B, Marques M, Ramalho-Santos J. Mitochondria functionality and sperm quality. Reproduction. 2013;146(5):R163–74.
Plaza Davila M, Martin Muñoz P, Tapia JA, Ortega Ferrusola C, Balao da Silva CC, Peña FJ. Inhibition of mitochondrial complex I leads to decreased motility and membrane integrity related to increased hydrogen peroxide and reduced ATP production, while the inhibition of glycolysis has less impact on sperm motility. PLoS One. 2015;10(9):e0138777.
Barbonetti A, Vassallo MR, Fortunato D, Francavilla S, Maccarrone M, Francavilla F. Energetic metabolism and human sperm motility: impact of CB1 receptor activation. Endocrinology. 2010;151(12):5882–92.
Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod. 2004;71:540–7.
Terrell KA, Wildt DE, Anthony NM, Bavister BD, Leibo SP, Penfold LM, et al. Evidence for compromised metabolic function and limited glucose uptake in spermatozoa from the teratospermic domestic cat (Felis catus) and cheetah (Acinonyx jubatus). Biol Reprod. 2010;83(5):833–41.
Hirano Y, Shibahara H, Obara H, Suzuki T, Takamizawa S, Yamaguchi C, et al. Relationships between sperm motility characteristics assessed by the computer-aided sperm analysis (CASA) and fertilization rates in vitro. J Assist Reprod Genet. 2001;18(4):213–8.
Kasai T, Ogawa K, Mizuno K, Nagai S, Uchida Y, Ohta S, et al. Relationship between sperm mitochondrial membrane potential, sperm motility, and fertility potential. Asian J Androl. 2002;4(2):97–103.
Travis AJ, Jorgez CJ, Merdiushev T, Jones BH, Dess DM, Diaz-Cueto L, et al. Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J Biol Chem. 2001;276(10):7630–6.
Hereng TH, Elgstøen KB, Cederkvist FH, Eide L, Jahnsen T, Skålhegg BS, et al. Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa. Hum Reprod. 2011;26(12):3249–63.
Shirwany NA, Zou MH. AMPK: a cellular metabolic and redox sensor. A minireview. Front Biosci (Landmark Ed). 2014;19:447–74.
Calder MD, Edwards NA, Betts DH, Watson AJ. Treatment with AICAR inhibits blastocyst development, trophectoderm differentiation and tight junction formation and function in mice. Mol Hum Reprod. 2017;23(11):771–85.
Singer D, Bretschneider HJ. Metabolic reduction in hypothermia: pathophysiological problems and natural examples--part 1. Thorac Cardiovasc Surg. 1990;38(4):205–11.
Galardo MN, Riera MF, Pellizzari EH, Cigorraga SB, Meroni SB. The AMP-activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-b-D-ribonucleoside, regulates lactate production in rat Sertoli cells. J Mol Endocrinol. 2007;39(4):279–88.
Swegen A, Lambourne SR, Aitken RJ, Gibb Z. Rosiglitazone improves stallion sperm motility, ATP content, and mitochondrial function. Biol Reprod. 2016;95(5):107;1–12.
Acknowledgments
The authors are thankful for Dr. Em-Orn Olanrattamanee for statistical analysis.
Funding
The present study was financially supported by (I) The Thailand Research Fund (Contract No. MRG 5980231) and (II) Research Unit of Reproductive Medicine and Fertility Preservation, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thuwanut, P., Comizzoli, P., Pruksananonda, K. et al. Activation of adenosine monophosphate–activated protein kinase (AMPK) enhances energy metabolism, motility, and fertilizing ability of cryopreserved spermatozoa in domestic cat model. J Assist Reprod Genet 36, 1401–1412 (2019). https://doi.org/10.1007/s10815-019-01470-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01470-5