Abstract
Purpose
Studies on humans and rodents have clearly shown that in vitro fertilization (IVF) is associated with abnormal placenta formation and function. Currently, dysregulated placental lipid metabolism is one of the emerging pathogenetic pathways implicated in adverse pregnancy outcomes. The purpose of this study was to identify the effects of IVF on lipid metabolism in the mouse placenta.
Methods
Two groups of mouse placentas, composed of control and IVF, were collected at embryonic day 18.5. Placental lipid profiles were measured using liquid chromatography coupled with mass spectrometry. The relative levels of individual lipid were examined and compared. The proteins and enzymes that regulate the phospholipid biosynthesis were also compared by western blot.
Results
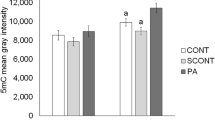
A significant increase in levels of phosphatidylcholines, phosphatidylethanolamines, phosphatidylinositols, phosphatidylglycerols, lysophosphatidylcholines, and mitochondrial cardiolipin were found in the IVF placenta. In addition, proteins and enzymes that regulate the phospholipid biosynthesis were also altered in IVF placentas.
Conclusions
After lipidomic analysis, we present the first detailed overview of the effect of IVF on lipid metabolism, especially phospholipid profiles in the placenta in a mouse model. The widespread lipidomic shifts identified in this study might explicate some of the placental dysfunction observed after IVF, thereby illustrating that phospholipids serve as early warning biomarkers of health risks in IVF offspring.






Similar content being viewed by others
References
Maltepe E, Fisher SJ. Placenta: the forgotten organ. Annu Rev Cell Dev Biol. 2015;31:523–52.
Holland O, Dekker Nitert M, Gallo LA, Vejzovic M, Fisher JJ, Perkins AV. Review: placental mitochondrial function and structure in gestational disorders. Placenta. 2017;54:2–9.
Morgan TK. Role of the placenta in preterm birth: a review. Am J Perinatol. 2016;33(3):258–66.
Hsiao EY, Patterson PH. Placental regulation of maternal-fetal interactions and brain development. Dev Neurobiol. 2012;72(10):1317–26.
Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol Rev. 2016;96(4):1509–65.
Haavaldsen C, Tanbo T, Eskild A. Placental weight in singleton pregnancies with and without assisted reproductive technology: a population study of 536,567 pregnancies. Hum Reprod. 2012;27(2):576–82.
Vrooman LA, Xin F, Bartolomei MS. Morphologic and molecular changes in the placenta: what we can learn from environmental exposures. Fertil Steril. 2016;106(4):930–40.
Zhang Y, Zhao W, Jiang Y, Zhang R, Wang J, Li C, et al. Ultrastructural study on human placentae from women subjected to assisted reproductive technology treatments. Biol Reprod. 2011;85(3):635–42.
Bloise E, Lin W, Liu X, Simbulan R, Kolahi KS, Petraglia F, et al. Impaired placental nutrient transport in mice generated by in vitro fertilization. Endocrinology. 2012;153(7):3457–67.
Collier AC, Miyagi SJ, Yamauchi Y, Ward MA. Assisted reproduction technologies impair placental steroid metabolism. J Steroid Biochem Mol Biol. 2009;116(1–2):21–8.
Chen S, Sun FZ, Huang X, Wang X, Tang N, Zhu B, et al. Assisted reproduction causes placental maldevelopment and dysfunction linked to reduced fetal weight in mice. Sci Rep. 2015;5:10596.
Rosenhouse-Dantsker A, Mehta D, Levitan I. Regulation of ion channels by membrane lipids. Compr Physiol. 2012;2(1):31–68.
Hresko RC, Kraft TE, Quigley A, Carpenter EP, Hruz PW. Mammalian glucose transporter activity is dependent upon anionic and conical phospholipids. J Biol Chem. 2016;291(33):17271–82.
Laganowsky A, Reading E, Allison TM, Ulmschneider MB, Degiacomi MT, Baldwin AJ, et al. Membrane proteins bind lipids selectively to modulate their structure and function. Nature. 2014;510(7503):172–5.
Dilworth MR, Sibley CP. Review: transport across the placenta of mice and women. Placenta. 2013;34(Suppl):S34–9.
Powell TL, Jansson T, Illsley NP, Wennergren M, Korotkova M, Strandvik B. Composition and permeability of syncytiotrophoblast plasma membranes in pregnancies complicated by intrauterine growth restriction. Biochim Biophys Acta. 1999;1420(1–2):86–94.
Omatsu K, Kobayashi T, Murakami Y, Suzuki M, Ohashi R, Sugimura M, et al. Phosphatidylserine/phosphatidylcholine microvesicles can induce preeclampsia-like changes in pregnant mice. Semin Thromb Hemost. 2005;31(3):314–20.
Lu YW, Claypool SM. Disorders of phospholipid metabolism: an emerging class of mitochondrial disease due to defects in nuclear genes. Front Genet. 2015;6:3.
Baig S, Lim JY, Fernandis AZ, Wenk MR, Kale A, Su LL, et al. Lipidomic analysis of human placental syncytiotrophoblast microvesicles in adverse pregnancy outcomes. Placenta. 2013;34(5):436–42.
Korkes HA, Sass N, Moron AF, Camara NO, Bonetti T, Cerdeira AS, et al. Lipidomic assessment of plasma and placenta of women with early-onset preeclampsia. PLoS One. 2014;9(10):e110747.
Brown SH, Eather SR, Freeman DJ, Meyer BJ, Mitchell TW. A lipidomic analysis of placenta in preeclampsia: evidence for lipid storage. PLoS One. 2016;11(9):e0163972.
Lam SM, Chua GH, Li XJ, Su B, Shui G. Biological relevance of fatty acyl heterogeneity to the neural membrane dynamics of rhesus macaques during normative aging. Oncotarget. 2016;7(35):55970–89.
Lam SM, Tong L, Duan X, Petznick A, Wenk MR, Shui G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J Lipid Res. 2014;55(2):289–98.
Shui G, Guan XL, Low CP, Chua GH, Goh JS, Yang H, et al. Toward one step analysis of cellular lipidomes using liquid chromatography coupled with mass spectrometry: application to Saccharomyces cerevisiae and Schizosaccharomyces pombe lipidomics. Mol BioSyst. 2010;6(6):1008–17.
Shui G, Cheong WF, Jappar IA, Hoi A, Xue Y, Fernandis AZ, et al. Derivatization-independent cholesterol analysis in crude lipid extracts by liquid chromatography/mass spectrometry: applications to a rabbit model for atherosclerosis. J Chromatogr A. 2011;1218(28):4357–65.
Santos JM, Kowluru RA. Role of mitochondria biogenesis in the metabolic memory associated with the continued progression of diabetic retinopathy and its regulation by lipoic acid. Invest Ophthalmol Vis Sci. 2011;52(12):8791–8.
Festing MF. Design and statistical methods in studies using animal models of development. ILAR J. 2006;47(1):5–14.
Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Phys Cell Physiol. 2007;292(1):C33–44.
Julienne CM, Tardieu M, Chevalier S, Pinault M, Bougnoux P, Labarthe F, et al. Cardiolipin content is involved in liver mitochondrial energy wasting associated with cancer-induced cachexia without the involvement of adenine nucleotide translocase. Biochim Biophys Acta. 2014;1842(5):726–33.
Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8(3):224–36.
Liu YY, Chen MB, Cheng L, Zhang ZQ, Yu ZQ, Jiang Q, et al. microRNA-200a downregulation in human glioma leads to Galphai1 over-expression, Akt activation, and cell proliferation. Oncogene. 2018;37(8):1119.
McMaster CR. From yeast to humans - roles of the Kennedy pathway for phosphatidylcholine synthesis. FEBS Lett. 2017;592(8):1256–72.
Saini-Chohan HK, Holmes MG, Chicco AJ, Taylor WA, Moore RL, McCune SA, et al. Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J Lipid Res. 2009;50(8):1600–8.
Lane M, Robker RL, Robertson SA. Parenting from before conception. Science. 2014;345(6198):756–60.
Luke B, Gopal D, Cabral H, Stern JE, Diop H. Adverse pregnancy, birth, and infant outcomes in twins: effects of maternal fertility status and infant gender combinations; the Massachusetts Outcomes Study of Assisted Reproductive Technology. Am J Obstet Gynecol. 2017;217(3):330–e1- e15.
Moessinger C, Klizaite K, Steinhagen A, Philippou-Massier J, Shevchenko A, Hoch M, et al. Two different pathways of phosphatidylcholine synthesis, the Kennedy pathway and the lands cycle, differentially regulate cellular triacylglycerol storage. BMC Cell Biol. 2014;15:43.
Rexhaj E, Paoloni-Giacobino A, Rimoldi SF, Fuster DG, Anderegg M, Somm E, et al. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. J Clin Invest. 2013;123(12):5052–60.
Seli E, Babayev E, Collins SC, Nemeth G, Horvath TL. Minireview: metabolism of female reproduction: regulatory mechanisms and clinical implications. Mol Endocrinol. 2014;28(6):790–804.
Prates EG, Nunes JT, Pereira RM. A role of lipid metabolism during cumulus-oocyte complex maturation: impact of lipid modulators to improve embryo production. Mediat Inflamm. 2014;2014:692067.
Fayezi S, Darabi M, Darabi M, Nouri M, Rahimipour A, Mehdizadeh A. Analysis of follicular fluid total phospholipids in women undergoing in-vitro fertilisation. J Obstet Gynaecol. 2014;34(3):259–62.
Bradley J, Pope I, Masia F, Sanusi R, Langbein W, Swann K, et al. Quantitative imaging of lipids in live mouse oocytes and early embryos using CARS microscopy. Development. 2016;143(12):2238–47.
Vilella F, Ramirez LB, Simon C. Lipidomics as an emerging tool to predict endometrial receptivity. Fertil Steril. 2013;99(4):1100–6.
Barrett HL, Dekker Nitert M, McIntyre HD, Callaway LK. Normalizing metabolism in diabetic pregnancy: is it time to target lipids? Diabetes Care. 2014;37(5):1484–93.
Baumann M, Korner M, Huang X, Wenger F, Surbek D, Albrecht C. Placental ABCA1 and ABCG1 expression in gestational disease: pre-eclampsia affects ABCA1 levels in syncytiotrophoblasts. Placenta. 2013;34(11):1079–86.
Dhalwani NN, Boulet SL, Kissin DM, Zhang Y, McKane P, Bailey MA, et al. Assisted reproductive technology and perinatal outcomes: conventional versus discordant-sibling design. Fertil Steril. 2016;106(3):710–6 e2.
Nicolson GL, Ash ME. Membrane lipid replacement for chronic illnesses, aging and cancer using oral glycerolphospholipid formulations with fructooligosaccharides to restore phospholipid function in cellular membranes, organelles, cells and tissues. Biochim Biophys Acta. 2017;1859(9 Pt B):1704–24.
van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, Jacobs RL. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta. 2017;1859(9 Pt B):1558–72.
Dumas JF, Goupille C, Julienne CM, Pinault M, Chevalier S, Bougnoux P, et al. Efficiency of oxidative phosphorylation in liver mitochondria is decreased in a rat model of peritoneal carcinosis. J Hepatol. 2011;54(2):320–7.
Babayev E, Seli E. Oocyte mitochondrial function and reproduction. Curr Opin Obstet Gynecol. 2015;27(3):175–81.
Choi S, Hedman AC, Sayedyahossein S, Thapa N, Sacks DB, Anderson RA. Agonist-stimulated phosphatidylinositol-3,4,5-trisphosphate generation by scaffolded phosphoinositide kinases. Nat Cell Biol. 2016;18(12):1324–35.
Viaud J, Mansour R, Antkowiak A, Mujalli A, Valet C, Chicanne G, et al. Phosphoinositides: important lipids in the coordination of cell dynamics. Biochimie. 2016;125:250–8.
Li B, Xiao X, Chen S, Huang J, Ma Y, Tang N, et al. Changes of phospholipids in fetal liver of mice conceived by in vitro fertilization. Biol Reprod. 2016;94(5):105.
Shimano H, Sato R. SREBP-regulated lipid metabolism: convergent physiology - divergent pathophysiology. Nat Rev Endocrinol. 2017;13(12):710–30.
Eberle D, Hegarty B, Bossard P, Ferre P, Foufelle F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 2004;86(11):839–48.
Shao W, Espenshade PJ. Expanding roles for SREBP in metabolism. Cell Metab. 2012;16(4):414–9.
Jeon TI, Osborne TF. SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol Metab. 2012;23(2):65–72.
Cornell RB, Ridgway ND. CTP:phosphocholine cytidylyltransferase: function, regulation, and structure of an amphitropic enzyme required for membrane biogenesis. Prog Lipid Res. 2015;59:147–71.
Acknowledgements
We are grateful to Guangzhou Shui and Sin Man Lam of the Key Laboratory of Molecular Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, for technical help. We also appreciate the valuable comments from other members of our laboratory.
Funding
This study was supported by the grants from the National Natural Science Foundation of China (31640056 and 31801250), Shaanxi Natural Science Foundation of China (2016JM8052), Key Research and Development Program of Shaanxi, China (2018SF-258), and Scientific and Technical Innovatory Project of Tangdu Hospital (2017LCYJ001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The present study was reviewed and approved by the Ethics Committee of Animal and Medicine of the Tangdu Hospital of The Fourth Military Medical University (approval identification: TDLL-2013051) and was conducted in accordance with the guidelines from the Committee on the Use of Live Animals in Teaching and Research of the Tangdu Hospital of The Fourth Military Medical University.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, S., Wang, J., Wang, M. et al. In vitro fertilization alters phospholipid profiles in mouse placenta. J Assist Reprod Genet 36, 557–567 (2019). https://doi.org/10.1007/s10815-018-1387-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1387-y