Abstract
Purpose
The neurokinin B (NKB)/NK3 receptor (NK3R) and kisspeptin (KISS1)/kisspeptin receptor (KISS1R), two systems essential for reproduction, are present in human granulosa cells (GCs) of healthy women and contribute to the control of fertility, at least partially, by acting on the gonads. However, little is known about the expression of these systems in GCs of women with polycystic ovarian syndrome (PCOS). The aim of this study was to analyze the expression of NKB/NK3R and KISS1/KISS1R in mural granulosa (MGCs) and cumulus cells (CCs) of PCOS women.
Methods
A cross-sectional study was performed in 46 healthy women and 43 PCOS women undergoing controlled ovarian stimulation. MGCs and CCs were collected from pre-ovulatory follicles after transvaginal ultrasound-guided oocyte retrieval and the expression of the genes encoding NKB (TAC3), NK3R (TACR3), KISS1, and its receptor (KISS1R) was analyzed using real-time quantitative RT-PCR.
Results
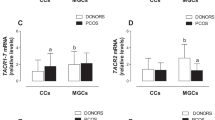
TAC3, TACR3, and KISS1 mRNA levels were decreased in MGCs and CCs of PCOS women. TAC3 positively correlated with KISS1 in MGCs of healthy women and TACR3 was positively associated with KISS1R in CCs from healthy women. These associations were not observed in PCOS women.
Conclusion
The NKB/NK3R and KISS1/KISS1R systems are dysregulated in MGCs and CCs of PCOS women. The lower expression of these systems in GCs could contribute to the abnormal follicle development and defective ovulation that characterize the pathogenesis of PCOS.


Similar content being viewed by others
References
Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–97.
Livadas S, Diamanti-Kandarakis E. Polycystic ovary syndrome: definitions, phenotypes and diagnostic approach. Front Horm Res. 2013;40:1–21.
El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G. Polycystic ovarian syndrome: an updated overview. Front Physiol. 2016;7:124.
Navarro VM, Tena-Sempere M. Neuroendocrine control by kisspeptins: role in metabolic regulation of fertility. Nat Rev Endocrinol. 2011;8:40–53.
Jayasena CN, Franks S. The management of patients with polycystic ovary syndrome. Nat Rev Endocrinol. 2014;10:624–36.
Ramezanali F, Ashrafi M, Hemat M, Arabipoor A, Jalali S, Moini A. Assisted reproductive outcomes in women with different polycystic ovary syndrome phenotypes: the predictive value of anti-Müllerian hormone. Reprod BioMed Online. 2016;32:503–12.
Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum Reprod Update. 2016;22:709–24.
Lv Y, Zhao SG, Lu G, Leung CK, Xiong ZQ, Su XW, et al. Identification of reference genes for qRT-PCR in granulosa cells of healthy women and polycystic ovarian syndrome patients. Sci Rep. 2017;7:6961.
Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol. 2013;14:141–52.
de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KISS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–6.
Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27.
Pintado CO, Pinto FM, Pennefather JN, Hidalgo A, Baamonde A, Sanchez T, et al. A role for tachykinins in female mouse and rat reproductive function. Biol Reprod. 2003;69:940–6.
Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–8.
Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27.
Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–89.
Hu G, Lin C, He M, Wong AO. Neurokinin B and reproductive functions: “KNDy neuron” model in mammals and the emerging story in fish. Gen Comp Endocrinol. 2014;208:94–108.
Clarke H, Dhillo WS, Jayasena CN. Comprehensive review on kisspeptin and its role in reproductive disorders. Endocrinol Metab. 2015;30:124–41.
Skorupskaite K, George JT, Veldhuis JD, Millar RP, Anderson RA. Interactions between neurokinin B and kisspeptin in mediating estrogen feedback in healthy women. J Clin Endocrinol Metab. 2016;101:4628–36.
Candenas L, Lecci A, Pinto FM, Patak E, Maggi CA, Pennefather JN. Tachykinins and tachykinin receptors: effects in the genitourinary tract. Life Sci. 2005;76:835–62.
Page NM. Neurokinin B and pre-eclampsia: a decade of discovery. Reprod Biol Endocrinol. 2010;8:4.
Lasaga M, Debeljuk L. Tachykinins and the hypothalamo-pituitary-gonadal axis: an update. Peptides. 2011;32:1972–8.
Satake H, Kawada T. Overview of the primary structure, tissue distribution, and functions of tachykinins and their receptors. Curr Drug Targets. 2006;7:963–74.
Gaytán F, Gaytán M, Castellano JM, Romero M, Roa J, Aparicio B, et al. KiSS-1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS-1 mRNA levels in a rat model of ovulatory dysfunction. Am J Physiol Endocrinol Metab. 2009;296:E520–31.
Cejudo Roman A, Pinto FM, Dorta I, Almeida TA, Hernández M, Illanes M, et al. Analysis of the expression of neurokinin B, kisspeptin and their cognate receptors, NK3R and KISS1R in the human female genital tract. Fertil Steril. 2012;97:1213–9.
García-Ortega J, Pinto FM, Fernández-Sánchez M, Prados N, Cejudo-Román A, Almeida TA, et al. Expression of neurokinin B/NK3 receptor and kisspeptin/KISS1 receptor in human granulosa cells. Hum Reprod. 2014;29:2736–46.
Qi X, Salem M, Zhou W, Sato-Shimizu M, Ye G, Smitz J, et al. Neurokinin B exerts direct effects on the ovary to stimulate estradiol production. Endocrinology. 2016;157:3355–65.
Gaytan F, Garcia-Galiano D, Dorfman MD, Manfredi-Lozano M, Castellano JM, Dissen GA, et al. Kisspeptin receptor haplo-insufficiency causes premature ovarian failure despite preserved gonadotropin secretion. Endocrinology. 2014;155:3088–97.
Bhattacharya M, Babwah AV. Kisspeptin: beyond the brain. Endocrinology. 2015;156:1218–27.
León S, Barroso A, Vázquez MJ, García-Galiano D, Manfredi-Lozano M, Ruiz-Pino F, et al. Direct actions of kisspeptins on GnRH neurons permit attainment of fertility but are insufficient to fully preserve gonadotropic axis activity. Sci Rep. 2016;6:19206.
Jayasena CN, Abbara A, Comninos AN, Nijher GM, Christopoulos G, Narayanaswamy S, et al. Kisspeptin-54 triggers egg maturation in women undergoing in vitro fertilization. J Clin Invest. 2014;124:3667–77.
Calder M, Chan YM, Raj R, Pampillo M, Elbert A, Noonan M, et al. Implantation failure in female Kiss1-/- mice is independent of their hypogonadic state and can be partially rescued by leukemia inhibitory factor. Endocrinology. 2014;155:3065–78.
Fernandois D, Na E, Cuevas F, Cruz G, Lara HE, Paredes AH. Kisspeptin is involved in ovarian follicular development during aging in rats. J Endocrinol. 2016;228:161–70.
Skorupskaite K, George JT, Veldhuis JD, Anderson RA. Neurokinin B regulates gonadotropin secretion, ovarian follicle growth, and the timing of ovulation in healthy women. J Clin Endocrinol Metab. 2018;103:95–104.
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25.
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–7.
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:1–11.
Castellano JM, Navarro VM, Fernández-Fernández R, Nogueiras R, Tovar S, Roa J, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–25.
Dudek M, Ziarniak K, Sliwowska JH. Kisspeptin and metabolism: the brain and beyond. Front Endocrinol. 2018;9:145.
Sánchez-Garrido MA, Ruiz-Pino F, Manfredi-Lozano M, Leon S, Garcia-Galiano D, Castaño JP, et al. Obesity-induced hypogonadism in the male: premature reproductive neuroendocrine senescence and contribution of Kiss1-mediated mechanisms. Endocrinology. 2014;155:1067–79.
Sánchez-Garrido MA, Ruiz-Pino F, Manfredi-Lozano M, Leon S, Heras V, Castellano JM, et al. Metabolic and gonadotropic impact of sequential obesogenic insults in the female: influence of the loss of ovarian secretion. Endocrinology. 2015;156:2984–98.
Xiao Y, Ni Y, Huang Y, Wu J, Grossmann R, Zhao R. Effects of kisspeptin-10 on progesterone secretion in cultured chicken ovarian granulosa cells from preovulatory (F1-F3) follicles. Peptides. 2011;32:2091–7.
Laoharatchatathanin T, Terashima R, Yonezawa T, Kurusu S, Kawaminami M. Augmentation of metastin/kisspeptin mRNA expression by the proestrous luteinizing hormone surge in granulosa cells of rats: implications for luteinization. Biol Reprod. 2015;93:15.
Castellano JM, Gaytan M, Roa J, Vigo E, Navarro VM, Bellido C, et al. Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology. 2006;147:4852–62.
Cielesh ME, McGrath BM, Scott CJ, Norman ST, Stephen CP. The localization of kisspeptin and kisspeptin receptor in the canine ovary during different stages of the reproductive cycle. Reprod Domest Anim. 2017;52:24–8.
George JT, Kakkar R, Marshall J, Scott ML, Finkelman RD, Ho TW, et al. Neurokinin B receptor antagonism in women with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101:4313–21.
Funding
This work was supported by a grant from the Ministerio de Economía y Competitividad (RTC-2014-1431-1), Spain, with joint financing by FEDER funds from the European Union.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Approval for this work was obtained from the institutional Ethics Committees of CSIC and Hospital Virgen Macarena (Sevilla, Spain) and all patients gave informed written consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Table S1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Blasco, V., Pinto, F.M., Fernández-Atucha, A. et al. Altered expression of the kisspeptin/KISS1R and neurokinin B/NK3R systems in mural granulosa and cumulus cells of patients with polycystic ovarian syndrome. J Assist Reprod Genet 36, 113–120 (2019). https://doi.org/10.1007/s10815-018-1338-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1338-7