Abstract
Purpose
The purpose of this study was to evaluate the effects of mitochondrial supplementation (MS) on early embryonic development and to assess the safety of MS treatments using induced pluripotent stem cells (iPSCs) as the mitochondrial donor.
Methods
In this study, we evaluated the effect of MS on early embryonic development using induced pluripotent stem cells (iPSCs) as the donor. Mouse zygotes were injected with either mitochondria from iPSCs or a vehicle solution. Several parameters were evaluated, including the rates of blastocyst formation and implantation, the weight of E13.5 embryos and placentas, the distribution of the donor mitochondrial DNA (mtDNA), and the pattern of methylation in the differentially methylated regions (DMRs) of the H19 and Snrpn genes.
Results
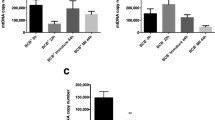
We found that neither the rates of blastocyst formation and implantation nor the weights of E13.5 embryos and placentas were significantly different between the MS and control groups. Additionally, the mtDNA from the iPSC donors could be detected in the muscle tissue of four fetuses and all placentas in the MS group. Finally, the methylation patterns of H19 and Snrpn DMRs remained unchanged by MS.
Conclusions
iPSC-derived mtDNA was directly involved in the process of embryonic development after MS. No adverse effects were seen when using iPSCs as a mitochondrial donor, but it remains to be seen whether this method can improve embryonic development, especially in older mice.




Similar content being viewed by others
References
Dyall SD, Brown MT, Johnson PJ. Ancient invasions: from endosymbionts to organelles. Science. 2004;304:253–7.
de Bruin JP, Dorland M, Spek ER, Posthuma G, van Haaften M, Looman CW, Te VE. Age-related changes in the ultrastructure of the resting follicle pool in human ovaries. Biol Reprod. 2004;70:419–24.
Bentov Y, Casper RF. The aging oocyte—can mitochondrial function be improved? Fertil Steril. 2013;99:18–22.
St John J. Ooplasm donation in humans: the need to investigate the transmission of mitochondrial DNA following cytoplasmic transfer. Hum Reprod. 2002;17:1954–8.
Seifer DB, DeJesus V, Hubbard K. Mitochondrial deletions in luteinized granulosa cells as a function of age in women undergoing in vitro fertilization. Fertil Steril. 2002;78:1046–8.
Perez GI, Trbovich AM, Gosden RG, Tilly JL. Mitochondria and the death of oocytes. Nature. 2000;403:500–1.
Yi YC, Chen MJ, Ho JY, Guu HF, Ho ES. Mitochondria transfer can enhance the murine embryo development. J Assist Reprod Genet. 2007;24:445–9.
Igarashi H, Takahashi T, Abe H, Nakano H, Nakajima O, Nagase S. Poor embryo development in post-ovulatoryin vivo-aged mouse oocytes is associated with mitochondrial dysfunction, but mitochondrial transfer from somatic cells is not sufficient for rejuvenation. Hum Reprod. 2016;31:2331–8.
Oktay K, Baltaci V, Sonmezer M, Turan V, Unsal E, Baltaci A, et al. Oogonial precursor cell-derived autologous mitochondria injection to improve outcomes in women with multiple IVF failures due to low oocyte quality: a clinical translation. Reprod Sci. 2015;22:1612–7.
Leighton PA, Ingram RS, Eggenschwiler J, Efstratiadis A, Tilghman SM. Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature. 1995;375:34–9.
Abu-Amero S, Monk D, Frost J, Preece M, Stanier P, Moore GE. The genetic aetiology of Silver-Russell syndrome. J Med Genet. 2008;45:193–9.
Leff SE, Brannan CI, Reed ML, Ozcelik T, Francke U, Copeland NG, Jenkins NA. Maternal imprinting of the mouse Snrpn gene and conserved linkage homology with the human Prader-Willi syndrome region. Nat Genet. 1992;2:259–64.
Han J, Yuan P, Yang H, Zhang J, Soh BS, Li P, et al. Tbx3 improves the germ-line competency of induced pluripotent stem cells. Nature. 2010;463:1096–100.
Hashizume O, Ohnishi S, Mito T, Shimizu A, Iashikawa K, Nakada K, et al. Epigenetic regulation of the nuclear-coded GCAT and SHMT2 genes confers human age-associated mitochondrial respiration defects. Sci Rep-UK. 2015;5:10434.
Ma H, Folmes CD, Wu J, Morey R, Mora-Castilla S, Ocampo A, et al. Metabolic rescue in pluripotent cells from patients with mtDNA disease. Nature. 2015;524(7564):234–8.
Hasuwa H, Muro Y, Ikawa M, Kato N, Tsujimoto Y, Okabe M. Transgenic mouse sperm that have green acrosome and red mitochondria allow visualization of sperm and their acrosome reaction in vivo. Exp Anim. 2010;59:105–7.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Pei Y, Yue L, Zhang W, Wang Y, Wen B, Zhong L, et al. Improvement in mouse iPSC induction by Rab32 reveals the importance of lipid metabolism during reprogramming. Sci Rep. 2015;5:16539.
Takeda K. Microinjection of cytoplasm or mitochondria derived from somatic cells affects parthenogenetic development of murine oocytes. Biol Reprod. 2005;72:1397–404.
Nagai S, Kasai T, Hirata S, Hoshi K, Yanagimachi R, Huang T. Cytoplasmic transfer in the mouse in conjunction with intracytoplasmic sperm injection. Reprod BioMed Online. 2004;8:75–80.
Yi Y, Chen M, Ho JY, Guu H, Ho ES. Mitochondria transfer can enhance the murine embryo development. J Assist Reprod Gen. 2007;24:445–9.
Luo SM, Ge ZJ, Wang ZW, Jiang ZZ, Wang ZB, Ouyang YC, et al. Unique insights into maternal mitochondrial inheritance in mice. Proc Natl Acad Sci. 2013;110:13038–43.
Cheng K, Fu X, Zhang R, Jia G, Hou Y, Zhu S. Effect of oocyte vitrification on deoxyribonucleic acid methylation of H19, Peg3, and Snrpn differentially methylated regions in mouse blastocysts. Fertil Steril. 2014;102:1183–90.
Hao Y, Wax D, Zhong Z, Murphy C, Ross JW, Rieke A, et al. Porcine skin-derived stem cells can serve as donor cells for nuclear transfer. Cloning Stem Cells. 2009;11:101–10.
Reddy P, Ocampo A, Suzuki K, Luo J, Bacman SR, Williams SL, et al. Selective elimination of mitochondrial mutations in the germline by genome editing. Cell. 2015;161:459–69.
Shimizu A, Mito T, Hayashi C, Ogasawara E, Koba R, Negishi I, et al. Transmitochondrial mice as models for primary prevention of diseases caused by mutation in the tRNA(Lys) gene. Proc Natl Acad Sci U S A. 2014;111:3104–9.
Yang L, Long Q, Liu J, Tang H, Li Y, Bao F, et al. Mitochondrial fusion provides an ‘initial metabolic complementation’ controlled by mtDNA. Cell Mol Life Sci. 2015;72:2585–98.
Motta PM, Nottola SA, Makabe S, Heyn R. Mitochondrial morphology in human fetal and adult female germ cells. Hum Reprod. 2000;15(Suppl 2):129–47.
Cagnone GL, Tsai TS, Makanji Y, Matthews P, Gould J, Bonkowski MS, et al. Restoration of normal embryogenesis by mitochondrial supplementation in pig oocytes exhibiting mitochondrial DNA deficiency. Sci Rep. 2016;6:23229.
Morrow EH, Reinhardt K, Wolff JN, Dowling DK. Risks inherent to mitochondrial replacement. EMBO Rep. 2015;16:541–4.
Lee HS, Ma H, Juanes RC, Tachibana M, Sparman M, Woodward J, et al. Rapid mitochondrial DNA segregation in primate preimplantation embryos precedes somatic and germline bottleneck. Cell Rep. 2012;1:506–15.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81370680, 81402168, and 31571497), the Specialized Research Fund for the Doctoral Program of the Chinese Ministry of Education (Grant No. 20130171130009), and the Natural Science Foundation’s Key Research Project of Guangdong Province (Grant No. 2013020012660).
Author information
Authors and Affiliations
Contributions
Ruiqi Li analyzed the data and drafted the manuscript. Bingqiang Wen induced iPSCs and evaluated the pluripotency of iPSCs. Haijing Zhao also drafted the manuscript. Nengyong Ouyang, Songbang O, and Meiqi Mai collected the data. Wenjun Wang revised the manuscript. Jianyong Han and Dongzi Yang conceived and designed the study. All authors interpreted the data.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
All animal protocols were approved by and performed in accordance with the requirements of the Institutional Animal Care and Use Committee at the authors’ university.
Electronic supplementary material
Supplementary Figure 1
The pluripotency detection of iPSCs. A) AP test; B) three germ layersof the teratoma; C) immunostaining of OCT4 and SOX2. (JPEG 8614 kb)
Supplementary Figure 2
Mitochondria ultrastructure and morphology of iPSCs. IPSCs mitochondria were immature, with round or oval shapes, possess few cristae and have an electron dense matrix. Mitochondria (arrow), nucleus (*) (12000×).. (JPEG 15112 kb)
Rights and permissions
About this article
Cite this article
Li, R., Wen, B., Zhao, H. et al. Embryo development after mitochondrial supplementation from induced pluripotent stem cells. J Assist Reprod Genet 34, 1027–1033 (2017). https://doi.org/10.1007/s10815-017-0948-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-0948-9