Abstract
Purpose
In this retrospective cohort study, we investigated the best embryo transfer strategy in ICSI cycles with ≤4 oocytes collected at oocyte retrieval.
Methods
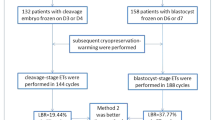
Women who underwent antagonist co-treatment COS for ICSI treatment between January 2010 and December 2015 at a private ART clinic (N = 2263). Eight hundred seventy-nine women (group 1) had ≤4 oocytes collected at oocyte retrieval, of whom 645 (group A) had cleavage stage embryo transfer (ET), and 234 (group B) had blastocyst ET. One thousand three hundred eighty-four women (group 2) had 10–15 oocytes collected at oocyte retrieval, of whom 676 (group C) had cleavage stage ET, and 708 women (group D) had blastocyst ET. Blastocyst vitrification was performed using the Cryotop method and FET using artificial cycles.
Results
In group 1, the cancellation rate was significantly lower in group A (25.2 vs 38 %). The pregnancy rate (PR), clinical PR, implantation rate (IR), and live birth rate (LBR) per ET and per oocyte retrieval were all lower in group A. The clinical PR, IR, and LBR per ET of vitrified-warmed blastocyst ET were significantly the highest. In group 2, the cycle cancellation rate was significantly lower in group C (3.5 vs 13.4 %). The PR, clinical PR, and IR per ET and per oocyte retrieval were all lower in group C. The LBR per ET was significantly lower, but the LBR per oocyte retrieval was not significantly lower in group C. Again, the PR, clinical PR, and IR per ET of vitrified-warmed blastocyst ET were significantly the highest.
Conclusions
Day 5 ET strategy has been reserved for normal or high responders. The improved pregnancy outcomes from blastocyst culture and cryopreservation may challenge ART to extend this benefit to poor responders.
Similar content being viewed by others
References
Garcia JE, Jones GS, Acosta AA, Wright G. HMG/hCG follicular maturation for oocytes aspiration: phase II, 1981. Fertil Steril. 1983;39:174–9.
Ferraretti AP, la Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitrofertilization: the Bologna criteria. Hum Reprod. 2011;26:1616–24.
Cobo A, de Los Santos MJ, Castello D, Gamiz P, Campos P, Remobi J. Outcomes of vitrified early cleavage-stage and blastocyst-stage embryos in a cryopreservation program: evaluation of 3150 warming cycles. Fertil Steril. 2012;98:1138–45.
Gardner DK, Vella P, Lane M, et al. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998;69:84–8.
Gardner D. Blastocyst culture: toward single embryo transfer. Hum Fertil. 2000;3:229–37.
Bungum M, Bungum L, Humaidan P, Andersen CY. Day 3 versus day 5 embryo transfer: a prospective randomized study. Reprod Biomed Online. 2003;7:98–104.
Frattarelli JL, Leondires MP, McKeeby JL, et al. Blastocyst transfer decreases multiple pregnancy rates in in vitro fertilization cycles: a randomized controlled trial. Fertil Steril. 2003;79:228–30.
Racowsky C, Combelles CMH, Nureddin A, et al. Day 3 and day 5 morphological predictors of embryo viability. Reprod Biomed Online. 2003;6:323–31.
Glujovsky D, Blake D, Farquhar C, Bardach A. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2012;7:CD002118.
Ata B, Kaplan B, Danzer H, Glassner M, Opsahl M, Tan SL, et al. Array CGH analysis shows that aneuploidy is not related with the number of embryos generated. Reprod Biomed Online. 2012;24:614–20.
Papanikolaou E, Kolibianakis E, Tournaye H, Venetis C, Fratemi H, Tarlatzis B, et al. Live birth rates after transfer of equal number of balstocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod. 2008;23:91–9.
Van der Auwera I, Debrock S, Spiessens C, Afschrift H, Bakelants E, Meuleman C, et al. A prospective randomized study, day 2 versus day 5 embryo transfer. Hum Reprod. 2002;17:1507–12.
Papanikolaou E, D’haeseleer E, Verheyen G, Van de Velde H, Camus M, Van Steirteghem A, et al. Live birth rate is significantly higher after blastocyst transfer when at least four embryos are available on day 3 of embryo culture. A randomized prospective study. Hum Reprod. 2005;20:3198–203.
Emiliani S, Delbaere A, Vannin AS, Biramane J, Verdoodt M, Englert Y, et al. Similar delivery rates in a selected group of patients, for day 2 and day 5 embryos both cultured in sequential medium: a randomized study. Hum Reprod. 2003;18:2145–50.
Kolb BA, Paulson RJ. The luteal phase of cycles utilizing controlled ovarian hyperstimulation and the possible impact of this hyperstimulation on embryo implantation. Am J Obstet Gynecol. 1997;176:1262–7.
Check JH, Choe JK, Katsoff D, Summers-Chase D, Wilson C. Controlled ovarian hyperstimulation adversely affects implantation after in vitro fertilization-embryo transfer. J Assist Reprod Genet. 1999;16:416–20.
Nikas G, Develioglu OH, Toner JP, Jones Jr HW. Endometrial pinopodes indicate a shift in the window of receptivity in IVF cycles. Hum Reprod. 1999;14:787–92.
Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Van Steirteghem A, et al. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil Steril. 2002;78:1025–9.
Papanikolaou EG, Bourgain C, Kolibianakis E, Tournaye H, Devroey P. Steroid receptor expression in late follicular phase endometrium in GnRH antagonist IVF cycles is already altered, indicating initiation of early luteal phase transformation in the absence of secretory changes. Hum Reprod. 2005;20:1541–7.
Horcajadas JA, Diaz-Gimeno P, Pellicer A, Simon C. Uterine receptivity and the ramifications of ovarian stimulation on endometrial function. Semin Reprod Med. 2007;25:454–60.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Ross R. Contrasting patterns in in vitro fertilization pregnancy rates among fresh autologous, fresh oocyte donor, and cryopreserved cycles with the use of day 5 or day 6 blastocysts may reflect differences in embryo-endometrium synchrony. Fertil Steril. 2008;89:20–6.
Haouzi D, Assou S, Mahmoud K, Tondeur S, Reme T, Hedon B, et al. gene expression profile of human endometrial receptivity: comparison between natural and stimulated cycles for the same patients. Hum Reprod. 2009;24:1436–45.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011a;96:344-8
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfers in high responders. Fertil Steril. 2011b;96:516-8
Polyzos NP, Devroey P. A systematic review of randomized trials for the treatment of poor ovarian responders: is there any light at the end of the tunnel? Fertil Steril. 2011;96:1058–61.
Demirol A, Gurgan T. Comparison of microdose flare-up and antagonist multiple-dose protocols for poor-responder patients: a randomized study. Fertil Steril. 2009;92:481–5.
Berkkanoglu M, Ozgur K. What is the optimum maximal gonadotropin dosage used in microdose flare-up cycles in poor responders? Fertil Steril. 2010;94:662–5.
Kuang Y, Chen Q, Hong Q, Lyu Q, Ai A, Fu Y, et al. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol). Reprod Biomed Online. 2014;29:684–91.
Ozcan Cenksoy P, Ficicioglu C, Kizilkale O, Suhha Bostanci M, Bakacak M, Yesiladali M, et al. The comparision of effect of microdose GnRH-a flare-up, GnRH antagonist/aromatase inhibitor letrozole and GnRH antagonist/clomiphene citrate protocols on IVF outcomes in poor responder patients. Gynecol Endocrinol. 2014;30:485–9.
Ubaldi F, Vaiarelli A, D’Anna R, Rienzi L. Management of poor responders in IVF: is there anything new? Biomed Res Int. 2014;2014:352098.
Polyzos NP, Nwoye M, Corona R, Blockeel C, Stoop D, Haentjens P, et al. Live birth rates in Bologna poor responders treated with ovarian stimulation for IVF/ICSI. Reprod BioMed Online. 2014;28:469–74.
Baker VL, Brown MB, Luke B, Smith GW, Ireland JJ. Gonadotropin dose is negatively correlated with live birth rate: analysis of more than 650,000 assisted reproductive technology cycles. Fertil Steril. 2015;104:1145–52.
Ozgur K, Berkkanoglu M, Bulut H, Isikli A, Coetzee K. Higher clinical pregnancy rates from frozen-thawed blastocyst transfers compared to fresh blastocyst transfers: a retrospective matched-cohort study. J Assist Reprod Genet. 2015a;32:1483-90
Ozgur K, Berkkanoglu M, Bulut H, Humaidan P, Coetzee K. Perinatal outcomes after fresh versus vitrified-warned blastocyst transfer: retrospective analysis. Fertil Steril 2015b;104:899-907
Ng EH, Yeung WS, So WW, Ho PC. An analysis of ectopic pregnancies following in vitro fertilisation treatment in a 10-year period. J Obstet Gynaecol. 1998;18:359–64.
Olivennes F, Fanchin R, Ledee N, Righini C, Kadoch IJ, Frydman R. Perinatal outcome and developmental studies on children born after IVF. Hum Reprod Update. 2002;8:117–28.
Kallen B, Finnstrom O, Nygren KG, Otterblad Olausson P, Wennerholm UB. In vitro fertilisation in Sweden: obstetric characteristics, maternal morbidity and mortality. BJOG. 2005;112:1529–35.
Lin CM, Chen CW, Chen PT, Lu TH, Li CY. Risks and causes of mortality among low-birthweight infants in childhood and adolescence. Paediat Perinat Epidemiol. 2007;21:465–72.
Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359:262–73.
Shih W, Rushford DD, Bourne H, Garrett C, McBain JC, Healy DL, et al. Factors affecting low birthweight after assisted reproduction technology: difference between transfer of fresh and cryopreserved embryos suggests an adverse effect of oocyte collection. Hum Reprod. 2008;23:1644–53.
Wennerholm UB, Soderstrom-Anttila V, Bergh C, Aittomaki K, Hazekamp J, Nygren KG, et al. Children born after cryopreservation of embryos or oocytes: a systematic review of outcome data. Hum Reprod. 2009;24:2158–72.
Pelkonen S, Koivunen R, Gissler M, Nuojua-Huttunen S, Suikkari AM, Hyden-Granskog C, et al. Perinatal outcome of children born after frozen and fresh embryo transfer: the Finnish cohort study 1995-2006. Hum Reprod. 2010;25:914–23.
Pinborg A, Loft A, Aaris Henningsen AK, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995-2006. Fertil Steril. 2010;94:1320–7.
Ishihara O, Kuwahara A, Saitoh H. Frozen-thawed blastocyst transfer reduces ectopic pregnancy risk: an analysis of single embryo transfer cycles in Japan. Fertil Steril. 2011;95:1966–9.
Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstet Gynecol. 2011;118:863–71.
Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366:1803–13.
Halliday JL, Ukoumunne OC, Baker HW, Breheny S, Jaques AM, Garrett C, et al. Increased risk of blastogenesis birth defects, arising in the first 4 weeks of pregnancy, after assisted reproductive technologies. Hum Reprod. 2010;25:59–65.
Kato O, Kawasaki N, Bodri D, Kuroda T, Kawachiya S, Kato K, et al. Neonatal outcome and birth defects in 6623 singletons born following minimal ovarian stimulation and vitrified versus fresh single embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2012;161:46–50.
Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2012;98:368–77.
Shapiro BS, Daneshmand ST, De Leon L, Garner FC, Aguirre M, Hudson C. Frozen-thawed embryo transfer is associated with a significantly reduced incidence of ectopic pregnancy. Fertil Steril. 2012;98:1490–4.
Imudia AN, Awonuga AO, Kaimal AJ, Wright DL, Styer AK, Toth TL. Elective cryopreservation of all embryos with subsequent cryothaw embryo transfer in patients at risk for ovarian hyperstimulation syndrome reduces the risk of adverse obstetric outcomes: a preliminary study. Fertil Steril. 2013;99:168–73.
Nakashima A, Araki R, Tani H, Ishihara O, Kuwahara A, Irahara M, et al. Implications of assisted reproductive technologies on term singleton birth weight: an analysis of 25,777 children in the national assisted reproduction registry of Japan. Fertil Steril. 2013;99:450–5.
Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Söderström-Anttila V, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update. 2013;19:87–104.
Sullivan EA, Zegers-Hochschild F, Mansour R, Ishihara O, de Mouzon J, Nygren KG, et al. International Committee for Monitoring Assisted Reproductive Technologies (ICMART) world report: assisted reproductive technology 2004. Hum Reprod. 2013;28:1375–90.
Wennerholm UB, Henningsen AK, Romundstad LB, Bergh C, Pinborg A, Skjaerven R, et al. Perinatal outcomes of children born after frozen thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Hum Reprod. 2013;28:2545–53.
Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril. 2014;101:128–33.
Evans J, Hannan NJ, Edgell TA, Vollenhoven BJ, Lutjen PJ, Osianlis T, et al. Fresh versus frozen embryo transfer: backing clinical decisions with scientific and clinical evidence. Hum Reprod Update. 2014;20:808–21.
Fauser BC, Devroey P. Reproductive biology and IVF: ovarian stimulation and luteal phase consequences. Trends Enocrinol Metab. 2003;14:236–42.
Humaidan P, Papanikolaou EG, Kyrou D, Alsbjerg B, Polyzos NP, Devroey P, et al. The luteal phase after GnRH-agonist triggering of ovulation: present and future perspectives. Reprod BioMed Online. 2012;24:134–41.
Zhu L, Li Y, Xu A. Influence of controlled ovarian hyperstimulation on uterine peristalsis in infertile women. Hum Reprod. 2012;27:2684–9.
Mainigi MA, Olalere D, Burd I, Sapienza C, Bartolomei M, Coutifaris C. Peri-implantation hormonal milieu: elucidating mechanisms of abnormal placentation and fetal growth. Biol Reprod. 2014;90:1–9.
Werner MD, Leondires MP, Schoolcraft WB, Miller BT, Copperman AB, Robins ED, et al. Clinically recognizable error rate after the transfer of comprehensive chromosomal screened euploid embryos is low. Fertil Steril. 2014;102:1613–8.
Grifo J, Kofinas J, Schoolcraft WB. Conceptions. Fertil Steril. 2014;102:658–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study received no financial support.
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
All participants in this study signed an informed consent (institutional Ethics committee form included).
Study data
The study data was partly presented at the 71st Annual Meeting of the ASRM, Baltimore, Maryland, USA, 17 to 21 October 2015.
Additional information
Capsule In a retrospective cohort study, day 5 blastocyst transfers were found to provide superior pregnancy rates compared to cleavage stage embryo transfers with further increases in pregnancy achieved through the transfer of vitrified-warmed blastocysts in frozen embryo transfers.
Rights and permissions
About this article
Cite this article
Berkkanoglu, M., Coetzee, K., Bulut, H. et al. Optimal embryo transfer strategy in poor response may include freeze-all. J Assist Reprod Genet 34, 79–87 (2017). https://doi.org/10.1007/s10815-016-0825-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0825-y