Abstract
Purpose
Cathepsin L and ADAMTS-1 are known to play critical roles in follicular rupture, ovulation, and fertility in mice. Similar studies in humans are limited; however, both are known to increase during the periovulatory period. No studies have examined either protease in the follicular fluid of women with unexplained infertility or infertility related to advanced maternal age (AMA). We sought to determine if alterations in cathepsin L and/or ADAMTS-1 existed in these infertile populations.
Methods
Patients undergoing in vitro fertilization (IVF) for unexplained infertility or AMA-related infertility were prospectively recruited for the study; patients with tubal or male factor infertility were recruited as controls. Follicular fluid was collected to determine gene expression (via quantitative polymerase chain reaction), enzyme concentrations (via enzyme-linked immunosorbent assays), and enzymatic activities (via fluorogenic enzyme cleavage assay or Western blot analysis) of cathepsin L and ADAMTS-1.
Results
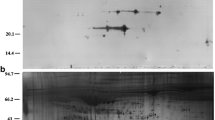
The analysis included a total of 42 patients (14 per group). We found no statistically significant difference in gene expression, enzyme concentration, or enzymatic activity of cathepsin L or ADAMTS-1 in unexplained infertility or AMA-related infertility as compared to controls. We also found no statistically significant difference in expression or concentration with advancing age.
Conclusions
Cathepsin L and ADAMTS-1 are not altered in women with unexplained infertility or AMA-related infertility undergoing IVF, and they do not decline with advancing age. It is possible that differences exist in natural cycles, contributing to infertility; however, our findings do not support a role for protease alterations as a common cause of infertility.






Similar content being viewed by others
References
Schochet SS. A suggestion as to the process of ovulation and ovarian cyst formation. A preliminary report. Anat Rec. 1916;10:447–57.
Robker RL, Russell DL, Espey LL, Lydon JP, O’Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci U S A. 2000;97:4689–94.
Brown HM, Dunning KR, Robker RL, Boerboom D, Pritchard M, Lane M, et al. ADAMTS1 cleavage of Versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol Reprod. 2010;83:549–57.
http://jaxmice.jax.org/strain/008352.html. Last accessed November 1, 2014.
García V, Kohen P, Maldonado C, Sierralta W, Muñoz A, Villarroel C, et al. Transient expression of progesterone receptor and cathepsin-l in human granulosa cells during the periovulatory period. Fertil Steril. 2012;97:707–13.
Yung Y, Maman E, Konopnicki S, Cohen B, Brengauz M, Lojkin I, et al. ADAMTS-1: a new human ovulatory gene and a cumulus marker for fertilization capacity. Mol Cell Endocrinol. 2010;328:104–8.
Freimann S, Ben-Ami I, Dantes A, Armon L, Ben Ya’cov-Klein A, Ron-El R, et al. Differential expression of genes coding for EGF-like factors and ADAMTS1 following gonadotropin stimulation in normal and transformed human granulosa cells. Biochem Biophys Res Commun. 2005;333:935–43.
Xiao S, Yubin L, Li T, Chen M, Xu Y, Wen Y, et al. Evidence for decreased expression of ADAMTS-1 associated with impaired oocyte quality in PCOS patients. JCEM. 2014;99:E1015–21.
Practice Committee of the American Society for Reproductive Medicine. Effectiveness and treatment for unexplained infertility. Fertil Steril. 2006;86:S111–4.
Qublan H, Amarin Z, Nawasreh M, Diab F, Malkawi S, Al-Ahmad N, et al. Luteinized unruptured follicle syndrome: incidence and recurrence rate in infertility women with unexplained infertility undergoing intrauterine insemination. Hum Reprod. 2006;21:2110–3.
Vollman RF. The menstrual cycle. In: Friedman E, editor. Major Problems in Obstetrics and Gynecology. Philadelphia: W.B. Saunders; 1977. p. 1–193.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8.
Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem. 2003;278:42330–9.
Sriraman V, Richards JS. Cathepsin L gene expression and promoter activation in rodent granulosa cells. Endocrinology. 2004;145:582–91.
Jansen E, Laven JS, Dommerholt HB, Polman J, van Rijt C, van den Hurk C, et al. Abnormal gene expression profiles in human ovaries from polycystic ovary syndrome patients. Mol Endocrinol. 2004;18:3050–63.
Acknowledgements
We would like to acknowledge the Division of Reproductive Endocrinology and Infertility and the University of Iowa’s Center for Advanced Reproductive Care for their support and assistance in sample collection. We would also like to thank Drs. Donna and Mark Santillan for their assistance in sample processing and use of materials from the University of Iowa REI Tissue bank. Finally, we would like to recognize Professor Miriam Bridget Zimmerman for her expertise in statistical analysis.
Conflict of interest
The authors declare that they have no conflict of interest.
Study funding
This work was supported by funding from the Departments of Pharmacology and Obstetrics and Gynecology at the University of Iowa.
Compliance with ethical standards
The authors declare that they have no conflict of interest. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
We performed a prospective, experimental study in IVF patients with unexplained infertility and advanced maternal age to determine if alterations in cathepsin L and/or ADAMTS-1 existed. We found no statistically significant difference in the gene expression, enzyme concentration, or enzymatic activity of either protease when compared to our control. Our findings do not support a role for protease alterations as a common cause of human infertility.
Rights and permissions
About this article
Cite this article
Cookingham, L.M., Van Voorhis, B.J. & Ascoli, M. Do alterations in follicular fluid proteases contribute to human infertility?. J Assist Reprod Genet 32, 737–745 (2015). https://doi.org/10.1007/s10815-015-0447-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0447-9