Abstract
Purpose
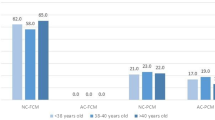
To analyze differences in morphokinetic parameters of chromosomally normal and aneuploid embryos utilizing time-lapse imaging and CGH microarray analysis.
Methods
This retrospective cohort study included patients undergoing IVF treatment and preimplantation genetic diagnosis for sex selection. A total of 460 embryos cultured in incubators with time-lapse imaging system (EmbryoScope) were selected for biopsy on day 3 of development. Subsequently, CGH microarray analysis was performed for aneuploidy screening of 24 chromosomes. Kinetic parameters including time for appearance of second polar body (tPB2), time of pronuclei appearance (tPNa), time of pronuclei fading (tPBf), time to division to 2(t2), 3(t3), 4(t4), 5(t5) cells, length of second and third cell cycle (CC2= t3 t2, CC3=t5-t3), synchrony of cell division from 2 to 4 cells (S2=t4-t3) and interval t5-t2 were analyzed to compare chromosomally normal and abnormal embryos.
Results
The mean time durations for tPNf, t2, t5, CC2, CC3, t5-t2 differed significantly between normal and abnormal embryos.
Conclusions
Time-lapse imaging morphokinetics may play a role in early prediction of aneuploid embryos due to differences in kinetic behavior that may aid in improving clinical outcome.
Similar content being viewed by others
References
Patrizio P, Bianchi V, Lalioti MD, Gerasimova T, Sakkas D. High rate of biological loss in assisted reproduction: it is in the seed, not in the soil. Reprod Biomed Online. 2007;14(1):92–5.
Bromer JG, Seli E. Assessment of embryo viability in assisted reproductive technology: shortcomings of current approaches and the emerging role of metabolomics. Curr Opin Obstet Gynecol. 2008;20(3):234–41.
Payne D, Flaherty SP, Barry MF, Matthews CD. Preliminary observations on polar body extrusion and pronuclear formation in human oocytes using time-lapse video cinematography. Hum Reprod. 1997;12(3):532–41.
Chamayou S, Patrizio P, Storaci G, Tomaselli V, Alecci C, Ragolia C, et al. The use of morphokinetic parameters to select all embryos with full capacity to implant. J Assist Reprod Genet. 2013;30(5):703–10. Apr 13.
Van Montfoort AP, Dumoulin JC, Kester AD, Evers JL. Early cleavage is a valuable addition to existing embryo selection parameters: a study using single embryo transfers. Hum Reprod. 2004;19(9):2103–8.
Cruz M, Garrido N, Herrero J, Pérez-Cano I, Muñoz M, Meseguer M. Timing of cell division in human cleavage-stage embryos is linked with blastocyst formation and quality. Reprod Biomed Online. 2012;25(4):371–81.
Yang Z, Liu J, Collins GS, Salem SA, Liu X. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5(1):24.
Meseguer M, Herrero J, Tejera A, et al. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26(10):2658.
Fragouli E, Wells D. Aneuploidy in the human blastocyst. Cytogen Genome Res. 2011;133(2–4):149–59.
Davies S, Christopikou D, Tsorva E, Kragianni A. Delayed cleavage division and prolonged transition between 2 and 4 cell stages in embryos identified as aneuploidy at 8 cell stage in array CGH. Hum Reprod. 2012;27:ii84–6.
Campbell A, Fishel S, Bowman N, Duffy S, Sedler M. Retrospective analysis of outcomes after IVF using an aneuploidy risk model derived from time-lapse imaging without PGS. Reprod Biomed Online. 2013;27(2):140–6.
Campbell A, Fishel S, Bowman N, Duffy S, Sedler M. Modelling a risk classification of aneuploidy in human embryos using non-invasive morphokinetics. Reprod Biomed Online. 2013;26(5):477–85.
Rubio I, Galan A, Larreategui Z, Ayerdi F . Controlled validation of embryo culture and selection by morphokinetic analysis : a randomized controlled trial of the embryoscope . Fertl Steril 2014, Sept 10 . (pubmed)
Hashimoto S, Kato N, Saeki K. Selection of high potential embryos for culture in polydimethyl siloxane microwells and time lapse imaging. Fertil Steril. 2012;97(2):332–7.
Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28(10):1115–21.
Le Caignec C, Spits C, Sermon K, De Rycke M, Thienpont B, Debrock S, et al. Single-cell chromosomal imbalances detection by array CGH. Nucleic Acids Res. 2006;34(9):e68.
Azzarello A, Hoest T, Mikkelsen A. The impact of time-lapse assessment on nuclearity: is multinucleation a proper character for embryo selection. Hum Reprod. 2012;27(2):ii103–5.
Lemmen JG, Agerholm I, Ziebe S. Kinetic markers of human embryo quality using time-lapse recordings of IVF/ICS fertilized oocytes. Reprod Biomed Online. 2008;17(3):385–91.
J. Stevens, M. Rawlins, A. Janesch, N. Treff, W.B. Schoolcraft . Time lapse observation of embryo development identifies later stage morphology based parameters associated with blastocyst quality but not chromosome constitution. Volume 98, Issue 3, Supplement, Page S30, September 2012
Semeniuk L, Mazur P, Mikitenko D, et al. Time lapse and aCGH, is there any connection between ploidy and embryo cleavage timing on early stages of embryo development. Fertil Steril. 2013;99(35):s6.
Basile N, Nogales Mdel C, Bronet F, Florensa M, Riqueiros M, Rodrigo L, et al. Increasing the probability of selecting chromosomally normal embryos by time-lapse morphokinetics analysis. Fertil Steril. 2014;101(3):699–704.
Bayram A, Ciray H, Sahin O, et al. Comparison of morphokinetic parameters between euploid and aneuploid embryos by time-lapse monitoring. Hum Reprod. 2012;27(2):ii103–5.
Friedman B, Chavez S, Behr B, et al. Non invasive imaging for the detection of human embryonic aneuploidy at the balstocyst stage. Fertil Steril. 2012;98(35 (suppl 3)):s38.
Author information
Authors and Affiliations
Corresponding author
Additional information
CapsuleDifferences in kinetic behavior were detected between normal and abnormal embryos during early embryonic development.
Rights and permissions
About this article
Cite this article
Chawla, M., Fakih, M., Shunnar, A. et al. Morphokinetic analysis of cleavage stage embryos and its relationship to aneuploidy in a retrospective time-lapse imaging study. J Assist Reprod Genet 32, 69–75 (2015). https://doi.org/10.1007/s10815-014-0372-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0372-3