Abstract
Purpose
Morphological assessment of human blastocysts has been effective for selecting embryos with high potential. However, they often show repeated shrinkage and expansion toward their hatching. Here we assessed whether capturing morphological changes over time of vitrified–warmed blastocysts could lead to a better selection of viable embryos from shrunken blastocysts.
Methods
The implantation rates of vitrified–warmed blastocysts that were shrunken or expanded (developing) at the time of loading for transfer were compared among 2,729 cycles that were subjected to single blastocyst transfer. Vitrified (107) and fresh blastocysts (17) were donated for the experimental study. To assess the relationship between morphology (expanded vs. shrunken) and the mitochondrial respiration of blastocysts, the oxygen consumption rate (OCR) was analyzed for 55 specimens using an uncoupler of oxidative phosphorylation. The remaining 69 blastocysts were used for recording morphological changes every 15 min for 48 h after warming.
Results
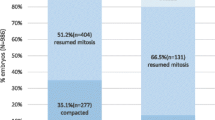
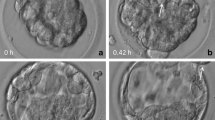
Because there were no surplus embryos, 7 % of the vitrified–warmed blastocysts were shrunken and transferred. The shrunken embryos had sufficient implantation ability (40 %). The OCR of the shrunken embryos was significantly lower than that of their expanded counterparts. Upon exposure to the uncoupler, the OCR of some shrunken embryos increased to levels similar to the expanded specimens. Time-lapse images revealed some shrunken embryos which formed blastocoel by 5 h following warming exhibited developmental competence to the hatched stage.
Conclusions
Data of the present study suggest a group of shrunken blastocysts contains many viable and clinically available embryos and time-lapse observation of vitrified–warmed blastocysts is a potential method to distinguish viable embryos from shrunken blastocysts.



Similar content being viewed by others
References
Wright VC, Chang J, Jeng G, Macaluso M. Assisted reproductive technology surveillance–United States, 2005. MMWR Surveill Summ. 2008;57:1–23.
Andersen AN, Goossens V, Ferraretti AP, Bhattacharya S, Felberbaum R, de Mouzon J, et al. Assisted reproductive technology in Europe, 2004: results generated from European registers by ESHRE. Hum Reprod. 2008;23:756–71.
Pandian Z, Templeton A, Serour G, Bhattacharya S. Number of embryos for transfer after IVF and ICSI: a Cochrane review. Hum Reprod. 2005;20:2681–7.
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–8.
Mio Y, Iwata K, Yumoto K, Iba Y. Several issues highlighted by time-lapse cinematography of human blastocyst during extended in vitro culture. In Japanese. J Mamm Ova Res. 2011;28:152–8.
Niimura S. Time-lapse videomicrographic analyses of contractions in mouse blastocysts. J Reprod Dev. 2003;49:413–23.
Yamanaka M, Hashimoto S, Amo A, Ito-Sasaki T, Abe H, Morimoto Y. Developmental assessment of human vitrified-warmed blastocysts based on oxygen consumption. Hum Reprod. 2011;26:3366–71.
Chen AA, Tan L, Suraj V, Reijo Pera R, Shen S. Biomarkers identified with time-lapse imaging: discovery, validation, and practical application. Fertil Steril. 2013;99:1035–43.
Cruz M, Garrido N, Herrero J, Perez-Cano I, Munoz M, Meseguer M. Timing of cell division in human cleavage-stage embryos is linked with blastocyst formation and quality. Reprod Biomed Online. 2012;25:371–81.
Hashimoto S, Kato N, Saeki K, Morimoto Y. Selection of high-potential embryos by culture in poly (dimethylsiloxane) microwells and time-lapse imaging. Fertil Steril. 2012;97:332–7.
Herrero J, Meseguer M. Selection of high potential embryos using time-lapse imaging: the era of morphokinetics. Fertil Steril. 2013;99:1030–4.
Hlinka D, Kalatova B, Uhrinova I, Dolinska S, Rutarova J, Rezacova J, et al. Time-lapse cleavage rating predicts human embryo viability. Physiol Res. 2012;61:513–25.
Pribenszky C, Matyas S, Kovacs P, Losonczi E, Zadori J, Vajta G. Pregnancy achieved by transfer of a single blastocyst selected by time-lapse monitoring. Reprod BioMed Online. 2010;21:533–6.
Kuwayama M, Vajta G, Leda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online. 2005;11:608–14.
Hashimoto S, Amo A, Hama S, Ito K, Nakaoka Y, Morimoto Y. Growth retardation in human blastocysts increases the incidence of abnormal spindles and decreases implantation potential after vitrification. Hum Reprod. 2013;28:1528–35.
Rehman KS, Bukulmez O, Langley M, Carr BR, Nackley AC, Doody KM, et al. Late stages of embryo progression are a much better predictor of clinical pregnancy than early cleavage in intracytoplasmic sperm injection and in vitro fertilization cycles with blastocyst-stage transfer. Fertil Steril. 2007;87:1041–52.
Shiku H, Shiraishi T, Ohya H, Matsue T, Abe H, Hoshi H, et al. Oxygen consumption of single bovine embryos probed by scanning electrochemical microscopy. Anal Chem. 2001;73:3751–8.
Checiu I, Checiu M. There are no in vivo pulsations of mouse blastocysts. Rom J Morphol Embryol. 1996;42:147–54.
Lin SP, Lee RK, Tsai YJ. In vivo hatching phenomenon of mouse blastocysts during implantation. J Assist Reprod Genet. 2001;18:341–5.
Acknowledgment
The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
Part of this work was supported by a grant from the Japan Society for the Promotion of Science (JPS-RFTF 23580397 to S.H.).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Time-lapse imaging paves the way for possible selection of viable blastocysts from shrunken embryos after vitrification.
Rights and permissions
About this article
Cite this article
Maezawa, T., Yamanaka, M., Hashimoto, S. et al. Possible selection of viable human blastocysts after vitrification by monitoring morphological changes. J Assist Reprod Genet 31, 1099–1104 (2014). https://doi.org/10.1007/s10815-014-0260-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0260-x