Abstract
Purpose
To investigate the association between SEPTIN12 gene variants and the risk of azoospermia caused by meiotic arrest.
Methods
Mutational analysis of the SEPTIN12 gene was performed using DNA from 30 Japanese patients with azoospermia by meiotic arrest and 140 fertile male controls.
Results
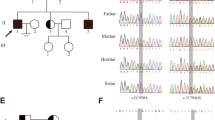
The frequencies of the c.204G>C (Gln38His) allele and the CC genotype were significantly higher in patients than in fertile controls (p < 0.05).
Conclusion
The c.204G>C (Gln38His) variant in the SEPTIN12 gene was associated with increased susceptibility to azoospermia caused by meiotic arrest.

Similar content being viewed by others
References
Reijo R, Lee TY, Salo P, Alagappan R, Brown LG, Rosenberg M, et al. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet. 1995;10:383–93.
Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathway. Nat Med. 2002;8 suppl 1:S41–9.
Miyamoto T, Hasuike S, Yogev L, Maduro MR, Ishikawa M, Westphal H, Lamb DJ. Azoospermia in patients heterozygous for a mutation in SYCP3. Lancet. 2003;362:1714–9.
Oliva R. Protamines and male infertility. Hum Reprod Update. 2006;12:417–35.
Dam AHDM, Koscinski I, Kremer JAM, Moutou C, Jaeger AS, Oudakker AR, Tournaye H, et al. Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia. Am J Hum Genet. 2007;81:813–20.
Dieterich K, Rifo RS, Faure AK, Hennebicq S, Amar BB, Zahi M, et al. Homozygous mutation of AURKC yields large-headed polyploidy spermatozoa and causes male infertility. Nat Genet. 2007;39:661–5.
Yatsenko AN, Roy A, Chen R, Ma L, Murthy LJ, Yan W, et al. Non-invasive genetic diagnosis of male infertility using spermatozoa RNA: KLHL10 mutations in oligozoospermic patients impair homodimerization. Hum Mol Genet. 2006;15:3411–9.
Yao G, Chen G, Pan T. Study of microdeletions in the Y chromosome of infertile men with idiopathic oligo- or azoospermia. J Assist Reprod Genet. 2001;18:612–6.
Ma J, Lu HY, Xia YK, Dong HB, Gu AH, Li ZY, et al. BCL2 Ala43Thr is a functional variant associated with protection against azoospermia in a Han-Chinese population. Biol Reprod. 2010;83:656–62.
Safarinejad MR, Shafiei N, Safarinejad S. The role of endothelial nitric oxide synthase (eNOS) T-786C, G894T, and 4a/b gene polymorphisms in the risk of idiopathic male infertility. Mol Reprod Dev. 2010;77:720–7.
Nasmyth K. Segregating sister genomes: the molecular biology of chromosome separation. Science. 2002;297:559–65.
Yoshida K, Kondoh G, Matsuda Y, Habu T, Nishimune Y, Morita T. The mouse RecA-like gene Dmc1 is required for homologous chromosome synapsis during meiosis. Mol Cell. 1998;1:707–18.
Pittman DL, Cobb J, Schimenti KJ, Wilson LA, Cooper DM, Brignull E, et al. Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell. 1998;1:697–705.
Edelmann W, Cohen PE, Kneitz B, Winand N, Lia M, Heyer J, et al. Mammalian MutS homologue 5 is required for chromosome pairing in meiosis. Nat Genet. 1999;21:123–7.
Baudat F, Manova K, Yuen JP, Jasin M, Keeney S. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell. 2000;6:989–98.
Kneitz B, Cohen PE, Avdievich E, Zhu L, Kane MF, Hou Jr H, et al. MutS homolog 4 localization to meiotic chromosomes is required for chromosome pairing during meiosis in male and female mice. Genes Dev. 2000;14:1085–97.
Romanienko PJ, Camerini-Otero RD. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell. 2000;6:975–87.
Yuan L, Liu JG, Zhao J, Brundell E, Daneholt B, Hoog C. The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol Cell. 2000;5:73–83.
Crackower MA, Kolas NK, Noguchi J, Sarao R, Kikuchi K, Kaneko H, et al. Essential role of Fkbp6 in male fertility and homologous chromosome pairing in meiosis. Science. 2003;300:1291–5.
Hayashi K, Yoshida K, Matsui Y. A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature. 2005;438:374–8.
Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, et al. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet. 2003;35:25–31.
Petukhova GV, Romanienko PJ, Camerini-Otero RD. The Hop2 protein has a direct role in promoting interhomolog interactions during mouse meiosis. Dev Cell. 2003;5:927–36.
Lin YH, Lin YM, Teng YN, Hsieh TY, Lin YS, Kuo PL. Identification of ten novel genes involved in human spermatogenesis by microarray analysis testicular tissue. Fertil Steril. 2006;86:1650–8.
Hall PA, Russell SEH. The pathobiology of the septin gene family. J Pathol. 2004;204:489–505.
Ding X, Yu W, Liu M, Shen S, Chen F, Wan B, Yu L. SEPT12 interacts with SEPT6 and this interaction alters the filament structure of SEPT6 in Hela cells. J Biochem Mol Biol. 2007;40:973–8.
Lin YH, Lin YM, Wang YY, Yu IS, Lin YW, Wang YH, et al. The expression level of Septin12 is critical for spermatogenesis. Am J Pathol. 2009;174:1857–68.
Lin YH, Chou CK, Hung YC, Yu IS, Pan HA, Lin SW, et al. SEPT12 deficiency causes sperm nucleus damage and developmental arrest of preimplantation embryos. Fertil Steril. 2011;95:363–5.
Miyakawa H, Miyamoto T, Koh E, Tsujimura A, Miyagawa Y, Saijo Y et al (2011) Single-nucleotide polymorphisms in the SEPTIN12 gene may be a genetic risk factor for Japanese patients with Sertoli cell-only syndrome. J Androl. doi:10.2164/jandrol.110.012146
Hung AJ, King P, Schlegel PN. Uniform testicular maturation arrest: a unique subset of men with nonobstructive azoospermia. J Urol. 2007;178:608–12.
Tsujimura A, Matsumiya K, Miyagawa Y, Tohda A, Miura H, Nishimura K, et al. Conventional multiple or microdissection testicular sperm extraction: a comparative study. Hum Reprod. 2002;17:2924–9.
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research (22591811, 22591812, and 23592388) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Grants
Grants-in-Aid for Scientific Research (22591811, 22591812, and 23592388) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Ministry of Health, Labour and Welfare of Japan.
Capsule c.204G>C (Gln38His) variant in the SEPTIN12 gene was associated with increased susceptibility to azoospermia by meiotic arrest in Japanese men.
Rights and permissions
About this article
Cite this article
Miyamoto, T., Tsujimura, A., Miyagawa, Y. et al. Single nucleotide polymorphisms in the SEPTIN12 gene may be associated with azoospermia by meiotic arrest in Japanese men. J Assist Reprod Genet 29, 47–51 (2012). https://doi.org/10.1007/s10815-011-9679-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-011-9679-5