Abstract
Purpose
The purpose of this study was to evaluate the effects of cryotop vitrification of sheep cumulus-oocyte complexes (COCs) on oocyte maturation, apoptotic gene expression and incidence of chromosomal abnormalities.
Methods
Freshly isolated (control group) and vitrified-warmed COCs (cryotop group) were matured in vitro. The expression of pro- and anti-apoptotic genes was investigated by real-time PCR. The incidence of numerical chromosomal abnormalities was evaluated by cytogenetic analysis.
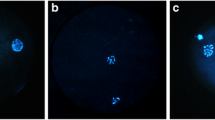
Results
The mean percentage of oocytes in the cryotop and control groups that reached metaphase II was 49.25 ± 3.01% and 51.94 ± 2.7% respectively. The expression rates of pro- and anti-apoptotic genes were similar in both groups, whereas the incidence of numerical chromosomal abnormalities was higher in the cryotop group compared to the control group (42.5% vs. 20%, p < 0.05).
Conclusion
Although cryotop vitrification of COCs did not affect the incidence of oocyte maturation or apoptotic gene expression, significant deficiencies in the maintenance of oocyte chromosomal organization were seen.



Similar content being viewed by others
References
Chen SU, Lien YR, Chao KH, Ho HN, Yang YS, Lee TY. Effects of cryopreservation on meiotic spindles of oocytes and its dynamis after thawing: clinical implications in oocyte freezing- a review article. Mol Cell Endocrinol. 2003;202:101–7.
Fabbri R, Porcu E, Marsella T, Primavera MR, Rocchetta G, Ciotti PM, et al. Technical aspects of oocyte cryopreservation. Mol Cell Endocrinol. 2000;169:39–42.
Vajta G, Kuwayama M. Improving cryopreservation systems. Theriogenology 2006;65:236–44.
Pegg DE. The role of vitrification techniques of cryopreservation in reproductive medicine. Hum Fertil. 2005;8:231–9.
Pukazhenthi BS, Comizzoli P, Travis AJ, Wildt DE. Applications of emerging to the study and conservation of threatened and endangered species. Reprod Fertil Dev. 2006;18:77–90.
Larman MG, Katz-Jaffe MG, Sheehan CB, Gardner DK. 1, 2 Propandiol and the type of cryopreservation adversely affect mouse oocyte physiology. Hum Reprod. 2006;22(1):250–9.
Jain JK, Paulson RJ. Oocyte cryopreservation. Fertil Steril. 2006;86 Suppl 3:1037–46.
Martino A, Songsasen N, Leibo SP. Development into blastocysts of bovine oocytes cryopreserved by ultra-rapid cooling. Biol Reprod. 1996;54:1059–69.
Vajta G, Holm P, Kuwayama M, Booth PJ, Jacobsen H. Open pulled straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Mol Reprod Dev. 1998;51:53–8.
Lane M, Scholcraft WB, Gardener DK. Vitrification of mouse and human blastocysts using a novel cryoloop container-less technique. Fertil Steril. 1999;72:1073–8.
Chian RC, Son WY, Huang JY, Cui SJ, Buckett WM, Tan SL. High survival rates and pregnancies of human oocytes following vitrification: preliminary report. Fertil Steril. 2005;84 Suppl 1:S36.
Kuwayama M, Kato O. All-around vitrification method for human oocytes and embryos. J Assist Reprod Genet. 2000;17:477.
Hinck H, Smissen PVD, Heusterpreute M, Donnay I, Hertogh RD, Pampfer S. Identification of caspase 3 and caspase-activated deoxyribonuclease in rat blastocysts and their implication in the induction of chromatin degradation (but not nuclear fragmentation) by high glucose. Biol Reprod. 2001;64:555–62.
Neuber E, Luetjens CM, Chan AWS, Schatten GP. Analysis of DNA fragmentation of in vitro cultured bovine blastocyts using TUNEL. Theriogenology 2002;57:2193–202.
Yang MY, Rajamahendran R. Expression of bcl-2 and Bax proteins in relation to quality of bovine oocytes and embryos produced in vitro. Anim Reprod Sci. 2002;70:159–69.
Men H, Monson RL, Parrish JJ, Rutledge JJ. Degeneration of cryopreserved bovine oocytes via apoptosis during subsequent culture. Cryobiology 2003;47:73–181.
Antonsson B, Martinou JC. The Bcl-2 protein family. Exp Cell Res. 2000;256:50–7.
Flaws JA, Hirshfield AN, Hewitt JA, Babus K, Furth PA. Effect of Bcl-2 on the primordial follicle endowment in the mouse ovary. Biol Reprod. 2001;64:1153–9.
Hussein MR. Apoptosis in the ovary: molecular mechanism. Hum Reprod Update. 2005;11(2):162–78.
Kim MR, Tilly JL. Current concepts in Bcl-2 family member regulation of female germ cell development and survival. Biochem Biophys Acta. 2004;1644:205–10.
Morita Y, Tilly JL. Oocyte apoptosis: like sand through an hourglass. Dev Biol. 1999;213:1–17.
Mockridge JW, Benton EC, Andreeva DS, Latchman MS, Marber MS, Heads RJ. IGF-1 regulates cardiac fibroblast apoptosis induced by osmotic stress. Biochem Biophys Res Commun. 2000;273:322–7.
Men H, Monson RL, Rutledge JJ. Effect of meiotic stages and maturation protocols on bovine oocytes resistance to cryopreservation. Theriogenology 2002;57(3):1095–103.
Albarracin JL, Morato R, Izquierdo D, Mogas T. Vitrification of calf oocytes: effects of maturation stage and prematuration treatment on the nuclear and cytoskeletal components of oocytes and their subsequent development. Mol Reprod Dev. 2005;72:239–49.
Boiso I, Marti M, Santalo J, Ponsa M, Barri PN, Veiga A. Confocal microscopy analysis of the spindle and chromosome configuration of human oocytes cryopreserved at the germinal vesicle and methaphase II stage. Hum Reprod. 2002;17:1885–91.
Ambrosini G, Andrisani A, Porcu E, Rebellato E, Revelli A, Caserta D, et al. Oocytes cryopreservation: state of art. Reprod Toxicol. 2006;22:250–62.
Zhou J, Shu HB, Joshi HC. Regulation of tubulin synthesis and cell cycle progression in mammalian cells by gamma-tubulin-mediated microtubule nucleation. J Cell Biochem. 2002;84:472–83.
Van der Elst J, Van den Abbeel E, Nerinckx S, Van Steirteghem A. Parthenogenetic activation pattern and microtubular organization of the mouse oocyte after exposure to 1, 2 propandiol. Cryobiology 1992;29:549–62.
Gook DA, Osborn SM, Johnston WIH. Cryopreservation of mouse and human oocytes using 1, 2 propandiol and the configuration of the meiotic spindle. Hum Reprod. 1993;8:1101–9.
Gosden RG. Prospects for oocyte banking and in vitro maturation. JNCI Monog. 2005;34:60–3.
Ebrahimi B, Valojerdi MR, Yazdi PE, Baharvand H, Farrokhi A. IVM and gene expression of sheep cumulus-oocyte complexes following vitrification by different methods. Reprod Biomed Online. 2010;20(1):26–34.
Keefer CL, Keystone A, Lazaris A, Bhatia B, Begin I, Bilodeau AS, et al. Production of cloned goat after nuclear transfer using adult somatic cells. Biol Reprod. 2002;66:199–203.
Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45.
Barekati Z, Gourabi H, Valojerdi MR, Yazdi PE. Previous maternal chemotherapy by cyclophosphaminde (CP) causes numerical chromosome abnormalities in preimplantation mouse embryos. Reprod Toxicol. 2008;26:278–81.
Gupta MK, Uhm SJ, Lee HT. Cryopreservation of immature and in vitro matured porcine oocytes by solid surface vitrification. Theriogenology 2007;67:238–48.
Genicot G, Leroy JL, Van Soom A, Donnay I. The use of a fluorescent dye, Nile red, to evaluate the lipid content of single mammalian oocytes. Theriogenology 2005;63:1181–94.
Pereira RM, Marques CC. Animal oocyte and embryo cryopreservation. Cell Tissue Banking. 2008;9:267–77.
Isachenko V, Isachenko E, Michelmann HW, Alabart JL, Vazquez I, Bezugly N, et al. Lipolysis and ultrastructure changes of intracellular vesicles after cooling of bovine and porcine GV oocytes. Anat Histol Embryol. 2001;30:333–8.
Miyake T, Kasai M, Zhu SE, Sakurai T, Machida T. Vitrification of mouse and embryos at various stages of development in an ethylene glycol-based solution by a simple method. Theriogenology 1993;40:121–4.
Vajta G. Oocytes and embryo vitrification. Annual ESDAR Conference 1999; 45–48.
Cetin Y, Bastan A. Cryopreservation of immature bovine oocytes by vitrification in straws. Anim Reprod Sci. 2006;92:29–36.
Shirazi A, Shams-Esfandabadi N, Hosseini SM, Karimi I. The presence of cumulus cells on nuclear maturation of sheep oocytes during in vitro maturation. Small Rumin Res. 2007;68:291–5.
Cuello C, Gill MA, Parrilla I, Tornel J, Vazquez JM, Roca J. In vitro development following one-step dilution of OPS-vitrified porcine blastocysts. Theriogenology 2004;62:1144–52.
Morita Y, Perez GI, Paris F, Miranda S, Ehleiter D, Haimovitz-Friedman A, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-1phosphate therapy. Nat Med. 2000;6:1109–14.
Mazoochi T, Salehnia M, Pourbeiranvand S, Forouzandeh M, Mowla SJ, Hajizadeh E. Analysis of apoptosis and expression of genes related to apoptosis in cultures of follicles derived from vitrified and non-vitrified ovaries. Mol Hum Reprod. 2009;15(3):155–64.
Anchamparuthy V, Pearson R, Gwazdauskas F. Expression pattern of apoptotic genes in vitrified-thawed bovine oocytes. Reprod Domest Anim 2009; Epub ahead of print
Succu S, Leoni GG, Berlinguer F, Madeddu M, Bebbere D, Mossa F, et al. Effect of vitrification solutions and cooling upon in vitro matured prepubertal ovine oocytes. Theriogenology 2007;68:107–14.
Succu S, Leoni GG, Bebbere D, Berlinguer F, Mossa F, Bogliolo L, et al. Vitrification devices affect structural and molecular status of in vitro matured ovine oocytes. Mol Reprod Dev. 2007;74:1337–44.
Chen SU, Lien YR, Cheng YY, Chen HF, Ho HN, Yang YS. Vitrification of mouse oocytes using closed pulled straws (CPS) achieves a high survival and preserves good patterns of meiotic spindles, compared with conventional straws, open pulled straws (OPS) and grids. Hum Reprod. 2001;16(11):2350–6.
Chen SU, Lien YR, Chen HF, Chao KH, Ho HN, Yang YS. Open pulled straws for vitrification of mature mouse oocytes preserve patterns of meiotic spindles and chromosomes better than conventional straws. Hum Reprod. 2000;15:2598–603.
Acknowledgements
This research was financially supported by Royan Institute. There is no conflict of interest in this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Apoptosis and chromosomal abnormality after oocyte vitrification.
Rights and permissions
About this article
Cite this article
Ebrahimi, B., Valojerdi, M.R., Eftekhari-Yazdi, P. et al. In vitro maturation, apoptotic gene expression and incidence of numerical chromosomal abnormalities following cryotop vitrification of sheep cumulus-oocyte complexes. J Assist Reprod Genet 27, 239–246 (2010). https://doi.org/10.1007/s10815-010-9401-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-010-9401-z