Abstract
Purpose
Oocyte cryopreservation may avoid many complications of human embryo freezing and provide future fertility for women undergoing cancer therapy. The objective of this study was to explore the application of intra- and extracellular sugars in combination with small amounts of dimethylsulfoxide (DMSO) to human oocyte cryopreservation as an alternative approach.
Methods
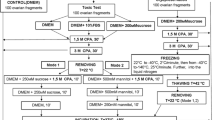
Discarded human oocytes that were obtained from IVF patients under informed consent and IRB approval, were cryopreserved by slow cooling to −196°C after being randomly distributed into three groups: (i) DMSO control without intra- and extracellular sugar; (ii) extracellular sugar (raffinose) + DMSO; and (iii) intra- and extracellular sugar (trehalose and raffinose, respectively) + DMSO. Subsequently, all cryopreserved oocytes were thawed rapidly, and their survival was assessed by morphological criteria after 24 h of culture.
Results
A total of 71 oocytes were evaluated in three groups with survival rates of 88.5% (23/26), 68.2% (15/22), and 52.2% (12/23) for intra- and extracellular sugar+DMSO, extracellular sugar+DMSO, and DMSO control groups, respectively.
Conclusion
These results support the use of intra- and extracellular sugars as an alternative approach for cryopreservation of human oocytes.


Similar content being viewed by others
References
Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986;1:884–6. doi:10.1016/S0140-6736(86) 90989-X.
Porcu E, Fabbri R, Seracchioli R, Ciotti PM, Magrini O, Flamigni C. Birth of a healthy female after intracytoplasmic sperm injection of cryopreserved human oocytes. Fertil Steril. 1997;68:724–6. doi:10.1016/S0015-0282(97) 00268-9.
Tucker MJ, Wright G, Morton PC, Massey JB. Birth after cryopreservation of immature oocytes with subsequent in vitro maturation. Fertil Steril. 1998;70:578–9. doi:10.1016/S0015-0282(98) 00205-2.
Yoon TK, Chung HM, Lim JM, Han SY, Ko JJ, Cha KY. Pregnancy and delivery of healthy infants developed from vitrified oocytes in a stimulated in vitro fertilization-embryo transfer program [letter]. Fertil Steril. 2000;74:180–1. doi:10.1016/S0015-0282(00) 00572-0.
Kuleshova L, Gianaroli L, Magli C, Ferraretti A, Trounson A. Birth following vitrification of a small number of human oocytes: case report. Hum Reprod. 1999;14:3077–9. doi:10.1093/humrep/14.12.3077.
Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11:300–8.
Borini A, Lagalla C, Bonu MA, Bianchi V, Flamigni C, Coticchio G. Cumulative pregnancy rates resulting from the use of fresh and frozen oocytes: 7 years’ experience. Reprod Biomed Online. 2006;12:481–6.
Boldt J, Tidswell N, Sayers A, Kilani R, Cline D. Human oocyte cryopreservation: 5-year experience with a sodium-depleted slow freezing method. Reprod Biomed Online. 2006;13:96–100.
Antinori M, Licata E, Dani G, Cerusico F, Versaci C, Antinori S. Cryotop vitrification of human oocytes results in high survival rate and healthy deliveries. Reprod Biomed Online. 2007;14:73–9.
Committee AP. Essential elements of informed consent for elective oocyte cryopreservation: a Practice Committee opinion. Fertil Steril. 2007;88:1495–6. doi:10.1016/j.fertnstert.2007.10.009.
Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril. 2006;86:70–80. doi:10.1016/j.fertnstert.2006.03.017.
Eroglu A, Russo MJ, Bieganski R, Fowler A, Cheley S, Bayley H, et al. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat Biotechnol. 2000;18:163–7. doi:10.1038/72608.
Eroglu A, Toner M, Toth TL. Beneficial effect of microinjected trehalose on the cryosurvival of human oocytes. Fertil Steril. 2002;77:152–8. doi:10.1016/S0015-0282(01) 02959-4.
Eroglu A, Bailey SE, Toner M, Toth TL. Successful cryopreservation of mouse oocytes by using low concentrations of trehalose and dimethylsulfoxide. Biol Reprod. 2009;80:70–8. doi:10.1095/biolreprod.108.070383.
Eroglu A, Lawitts JA, Toner M, Toth TL. Quantitative microinjection of trehalose into mouse oocytes and zygotes, and its effect on development. Cryobiology. 2003;46:121–34. doi:10.1016/S0011-2240(03) 00018-X.
McWilliams RB, Gibbons WE, Leibo SP. Osmotic and physiological responses of mouse zygotes and human oocytes to mono- and disaccharides. Hum Reprod. 1995;10:1163–71.
Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annu Rev Physiol. 1992;54:579–99. doi:10.1146/annurev.ph.54.030192.003051.
Potts M. Desiccation tolerance of prokaryotes. Microbiol Rev. 1994;58:755–805.
Beattie GM, Crowe JH, Lopez AD, Cirulli V, Ricordi C, Hayek A. Trehalose: a cryoprotectant that enhances recovery and preserves function of human pancreatic islets after long-term storage. Diabetes. 1997;46:519–23. doi:10.2337/diabetes.46.3.519.
Crowe JH, Crowe LM, Carpenter JF. Preserving dry biomaterials: the water replacement hypothesis, Part I. BioPharm. 1993;28:31.
Crowe JH, Crowe LM, Carpenter JF. Preserving dry biomaterials: the water replacement hypothesis, Part II. BioPharm. 1993;28:40–4.
Crowe JH, Leslie SB, Crowe LM. Is vitrification sufficient to preserve liposomes during freeze-drying? Cryobiology. 1994;31:355–66. doi:10.1006/cryo.1994.1043.
Somero GN. Protons, osmolytes, and fitness of internal milieu for protein function. Am J Physiol. 1986;251:R197–213.
Singer MA, Lindquist S. Thermotolerance in Saccharomyces cerevisiae: the Yin and Yang of trehalose. Trends Biotechnol. 1998;16:460–8. doi:10.1016/S0167-7799(98) 01251-7.
Sola-Penna M, Ferreira-Pereira A, Lemos AP, Meyer-Fernandes JR. Carbohydrate protection of enzyme structure and function against guanidinium chloride treatment depends on the nature of carbohydrate and enzyme. Eur J Biochem. 1997;248:24–9. doi:10.1111/j.1432-1033.1997.00024.x.
Chen Q, Haddad GG. Role of trehalose phosphate synthase and trehalose during hypoxia: from flies to mammals. J Exp Biol. 2004;207:3125–9. doi:10.1242/jeb.01133.
Johnson MH, Pickering SJ. The effect of dimethylsulphoxide on the microtubular system of the mouse oocyte. Development. 1987;100:313–24.
Vanderelst J, Vandenabbeel E, Nerinckx S, Vansteirteghem A. Parthenogenetic Activation Pattern and Microtubular Organization of the Mouse Oocyte after Exposure to 1, 2-Propanediol. Cryobiology. 1992;29:549–62. doi:10.1016/0011-2240(92) 90060-F.
Eroglu A, Elliott G, Wright DL, Toner M, Toth TL. Progressive elimination of microinjected trehalose during mouse embryonic development. Reprod Biomed Online. 2005;10:503–10.
Huang JYJ, Chen HY, Tan SL, Chian RC. Effects of osmotic stress and cryoprotectant toxicity on mouse oocyte fertilization and subsequent embryonic development in vitro. Cell Preserv Technol. 2006;4:149–60. doi:10.1089/cpt.2006.4.149.
Larman MG, Katz-Jaffe MG, Sheehan CB, Gardner DK. 1, 2-propanediol and the type of cryopreservation procedure adversely affect mouse oocyte physiology. Hum Reprod. 2007;22:250–9. doi:10.1093/humrep/del319.
Sher G, Keskintepe L, Mukaida T, Keskintepe M, Ginsburg M, Agca Y, et al. Selective vitrification of euploid oocytes markedly improves survival, fertilization and pregnancy-generating potential. Reprod Biomed Online. 2008;17:524–9.
Keskintepe L, Agca Y, Sher G, Keskintepe M, Maassarani G. High survival rate of metaphase II human oocytes after first polar body biopsy and vitrification: determining the effect of previtrification conditions. Fertil Steril. 2008.
Cobo A, Kuwayama M, Perez S, Ruiz A, Pellicer A, Remohi J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril. 2008;89:1657–64. doi:10.1016/j.fertnstert.2007.05.050.
Acknowledgments
The authors wish to thank IVF nurse/coordinator Cynthia Clower and Deanna Nelsen for clinical research assistance. This study was partially supported by grant R01HD049537 from the National Institute of Child Health and Human Development to A.E.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Younis, A., Carnovale, D., Butler, W. et al. Application of intra- and extracellular sugars and dimethylsulfoxide to human oocyte cryopreservation. J Assist Reprod Genet 26, 341–345 (2009). https://doi.org/10.1007/s10815-009-9316-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-009-9316-8