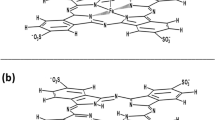
The interaction of indotricarbocyanine dyes with lipid-transfer protein Ns-LTP1 in Dulbecco's phosphate-buffered saline (PBS) was studied. An orthophenylene bridge with a chlorine in the meso-position of the dye was shown to be ineffective as a linker for protein macromolecules without a free cysteine residue. The indotricarbocyanine dye was modified into the N-hydroxysuccinimide ester to obtain a complex of the indotricarbocyanine dye with the Ns-LTP1 protein. A complex of dye molecules with the Ns-LTP1 protein was obtained by mixing a two-phase system of the protein in PBS and the dye activated ester in 1,2-dichloroethane. The half-width of the absorption spectrum increased from 56 to 61 nm; the fluorescence decay time, from 0.3 to 0.5 ns; and the degree of polarization, from 27 to 31% upon formation of the complex. It was established that HCl at concentrations equivalent to its content in gastric juice (0.1–0.5%) led to irreversible destruction of the dye. Capsules that can withstand stomach acid and dissolve in the intestines could be used to treat erosive and ulcerative lesions with peroral administration of the complex.
Similar content being viewed by others
References
P. I. Tolstykh, V. A. Derbenev, I. Yu. Kuleshov, A. M. Azimshoev, O. B. Tamrazova, A. S. Romanova, T. I. Karakhanov, and A. B. Solov′eva, Khirurgiya Zh. im. N. I. Pirogova, No. 12, 17–22 (2010).
A. V. Ishchuk and S. I. Leonovich, Nov. Khir., 16, No. 1, 44–54 (2008).
T. Dai, Y. Y. Huang, and M. R. Hamblin, Photodiagn. Photodyn. Ther., 6, Nos. 3–4, 170–188 (2009); doi: 10.1016/j. pdpdt.2009.10.008.
R. E. Saxton, M. Huang, and C. Foote, Proc. Annu. Meet. Am. Assoc. Cancer Res., 34, A2131 (1993).
G. S. Lipshutz, D. J. Castro, R. E. Saxton, R. P. Haugland, and J. Soudant, Laryngoscope, 104, No. 8, 996–1002 (1994); doi: https://doi.org/10.1288/00005537-199408000-00014.
A. R. Oseroff, D. Ohuoha, G. Ara, D. McAuliffe, J. Foley, and L. Cincotta, Proc. Natl. Acad. Sci. USA, 83, 9729–9733 (1986); doi: https://doi.org/10.1073/pnas.83.24.9729.
E. Delaey, F. van Laar, D. De. Vos, A. Kamuhabwa, P. Jacobs, and P. de Witte, J. Photochem. Photobiol., B, 55, 27–36 (2000); doi: https://doi.org/10.1016/S1011-1344(00)00021-X.
Y. P. Istomin, E. N. Alexandrova, E. A. Zhavrid, E. S. Voropay, M. P. Samtsov, K. N. Kaplevsky, A. P. Lugovsky, and A. Lugovsky, Exp. Oncol., 28, No. 1, 80–82 (2006).
M. P. Samtsov, E. S. Voropay, K. N. Kaplevsky, D. G. Melnikau, L. S. Lyashenko, and Yu. P. Istomin, J. Appl. Spectrosc., 76, 547–553 (2009).
M. Levitus and S. Ranjit, Q. Rev. Biophys., 44, No. 1, 123–151 (2011); doi: https://doi.org/10.1017/S0033583510000247.
R. K. Pandey, N. James, Y. Chen, and M. P. Dobhal, in: Topics in Heterocyclic Chemistry, Vol. 14, (2008), pp. 41–74; doi: https://doi.org/10.1007/7081_2008_113.
A. Thibon and V. C. Pierre, Anal. Bioanal. Chem., 394, 107–120 (2009); doi: https://doi.org/10.1007/s00216-009-2683-2.
H. E. Rajapakse, D. R. Reddy, S. Mohandessi, N. G. Butlin, and L. W. Miller, Angew. Chem., Int. Ed., 48, 4990–4992 (2009); doi: https://doi.org/10.1002/anie.200900858.
E. S. Voropai, M. P. Samtsov, A. P. Lugovskii, A. A. Lugovskii, T. S. Ermakova, L. P. Titov, and T. N. Bakaeva, Zdravookhranenie, No. 11, 45–47 (2006).
L. M. Lichtenberger, L. A. Graziani, E. J. Dial, B. D. Butler, and B. A. Hills, Science, 219, No. 4590, 1327–1329 (1983); doi: https://doi.org/10.1126/science.6828859.
Yu. I. Oshchepkova, O. N. Veshkurova, E. A. Rogozhin, A. Kh. Musolyamov, A. N. Smirnov, T. I. Odintsova, Ts. A. Egorov, E. V. Grishin, and Sh. I. Salikhov, Bioorg. Khim., 35, No. 3, 344–349 (2009).
D. A. Amanlikova and Yu. I. Oshchepkova, Khim.-Farm. Zh., 55, No. 7, 25–29 (2021); doi: https://doi.org/10.30906/0023-1134-2021-55-7-25-29.
E. S. Voropay, M. P. Samtsov, A. P. Lugovsky, E. A. Zhavrid, E. N. Alexandrova, Yu. P. Istomun, and I. N. Zhuravkin, Exp. Oncol., 19, 56–60 (1997).
D. S. Tarasov, M. P. Samtsov, and N. N. Krasnoperov, Zh. Beloruss. Gos. Univ. Fiz., No. 2, 4–11 (2022); doi: https://doi.org/10.33581/2520-2243-2022-2-4-11.
D. S. Tarasov, M. P. Samtsov, I. I. Khludeev, E. V. Malyushkova, and I. V. Semak, Zh. Prikl. Spektrosk., 89, No. 5, 605–613 (2022); doi: https://doi.org/10.47612/0514-7506-2022-89-5-605-613.
M. P. Samtsov, D. S. Tarasov, E. V. Malyushkova, I. I. Khludeev, A. P. Lugovskii, and I. V. Semak, Aktual. Vopr. Biol. Fiz. Khim., 6, No. 3, 499–504 (2021).
P. L. Southwick, L. A. Ernst, E. W. Tauriello, S. R. Parker, R. B. Mujumdar, S. R. Mujumdar, H. A. Clever, and S. Waggoner, Cytometry: J. Int. Soc. Anal. Cytol., 11, No. 3, 418–430 (1990); doi: https://doi.org/10.1002/cyto.990110313.
S. R. Mujumdar, R. B. Mujumdar, C. M. Grant, and A. S. Waggoner, Bioconjugate Chem., 7, No. 3, 356–362 (1996); doi: https://doi.org/10.1021/bc960021b.
A. J. Gordon and R. A. Ford, The Chemist's Companion, Wiley-Interscience, New York (1972).
N. V. Bel′ko, M. P. Samtsov, and A. P. Lugovskii, Zh. Beloruss. Gos Univ. Fiz., No. 2, 19–27 (2020); doi: https://doi.org/10.33581/2520-2243-2020-2-19-27.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 91, No. 2, pp. 273–280, March– April, 2024.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tarasov, D.S., Samtsov, M.P., Oshchepkova, Y.I. et al. Photophysical Properties of Indotricarbocyanine Dyes Complexed with Lipid-Transfer Protein Ns-LTP1. J Appl Spectrosc 91, 349–356 (2024). https://doi.org/10.1007/s10812-024-01727-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-024-01727-7