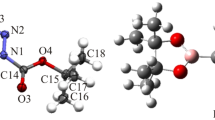
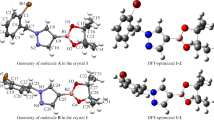
To better understand the molecular definition of 3-[4-(trifluoromethyl)phenyl]-3a,4,8,8a-tetrahydro-6H-[1,3] dioxepino[5,6-d][1,2]oxazole (OXE–OXA) compound, we examined its molecular geometric structure and spectroscopic properties in detail. First, we determined the OXE–OXA compound’s crystal structure using single-crystal X-ray diffraction data, then we grew a single crystal of the OXE–OXA compound using the slow evaporation solution magnification technique at room temperature with ethanol. It was found that the OXE–OXA compound crystallizes in the monoclinic crystal system with the noncentrosymmetric space group P1 21/n1. We performed the theoretical calculations for OXE–OXA compound at the B3LYP/6-311++G(d,p) and HSEh1PBE/6-311++G(d,p) levels of the density functional theory method. According to the comparison of our obtained data, the experimental 1H and 13C nuclear magnetic resonance chemical shifts were in strong agreement with the values for simulated chemical shifts. Later, we investigated the experimental FT-IR and theoretical IR spectrum of OXE–OXA compounds in the 4000–400 cm–1 region.
Similar content being viewed by others
References
B. Prugoveki, M. Marinkovi, M. Vinkovi, and M. Dumi, Croatica Chem. Acta C, 79, 219–226 (2006).
W. Gul, P. Carvalho, A. Galal, M. A. Averyb, and M. A. El Sohly, Acta Cryst., 65, 358–359 (2009).
D. Palla, A. I. Antoniou, M. Baltas, C. Menendez, P. Grellier, E. Mouray, and C. M. Athanassopoulos, Molecules, 25, Article ID 4858 (2020).
C. H. Lakshmi Praveena, V. Esther Rani, Y. N. Spoorthy, and L. K. Ravindranath, J. Chem. Pharm. Res., 5, 280–292 (2013).
R. S. Pavelyev, R. M. Vafina, K. V. Balakin, O. I. Gnezdilov, A. B. Dobrynin, O. A. Lodochnikova, R. Z. Musin, G. A. Chmutova, S. A. Lisovskaya, and L. E. Nikitina, Hindawi J. Chem., 14, Article ID 3589342 (2018).
E. Rani, M. Rani, and L. K. Ravindranath, J. Med. Org. Chem., 3, 11–20 (2016).
S. Jana, S. Iram, J. Thomas, M. Q. Hayat, C. Pannecouque, and W. Dehaen, Molecules, 22, 303 (2017).
H. Deng, X. Huang, C. Jin, C. M. Jin, and Z. S. Quan, Bioorg. Chem., 94, Article ID 103467 (2020).
N. T. Giang, D. T. Tuyet Anh, H. T. Phuong, N. Thanh, and N. Tuyen, Vietnam J. Chem., 56, 167–171 (2018).
K. V. Chikkula and S. Raja, Int. J. Pharm. Pharm. Sci., 9, 13–24 (2017).
A. Shaik, R. R. Bhandare, K. Palleapati, S. Nissankararao, V. Kancharlapalli, and Shahanaaz Shaik, Molecules, 25, 1047 (2020).
A. Banerjee, S. Bandyopadhyay, A. Gayen, T. Sengupta, A. Das, G. K. Chatterjee, and S. K. Chaudhuri, Arzneimittelforschung, 44, 863–866 (1994).
K. V. Chikkula and R. Sundararajan, Med. Chem. Res., 26, 3026–3037 (2017).
R. Chaithra, S. Kumar, T. Pramila, and D. N. Vidya, EJPMR, 6, 274–281 (2019).
Y. Kara and S. Yalduz, J. Mol. Struct., 1193, 158–165 (2019).
APEX2, version 2014.11-0, Bruker (2014) Bruker AXS Inc., Madison
SAINT, version 8.34A, Bruker (2013) Bruker AXS Inc., Madison
SADABS, version 2014/5, Bruker (2014) Bruker AXS Inc., Madison
G. M. Sheldrick, Acta Crystallogr. A, A71, 3 (2015).
G. M. Sheldrick, Acta Crystallogr. C, C71, 3 (2015).
O. V. Dolomanov, L. Bourhis, R. J. Gildea, A. K. Howard, and H. Puschmann, J. Appl. Crystallogr., 42, 339–341 (2009).
A. L. Spek, J. Appl. Crystallogr., 36, 7–11 (2003).
C. F. Macrae and I. Sovago, J. Appl. Crystallogr., 53, 226 (2019).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Fox, Gaussian 09, Revision A.1, Gaussian, Inc., Wallingford CT (2009).
GaussView, Version 5, Roy Dennington, Todd Keith, and John Millam, Semichem Inc., Shawnee Mission KS (2009).
A. D. Becke, J. Chem. Phys., 98, Article ID 5648 (1993).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B, 37, 785 (1988).
J. Heyd and G. Scuseria, J. Chem. Phys., 121, Article ID 1187 (2004).
J. Heyd and G. E. Scuseria, J. Chem. Phys., 120, Article ID 7274 (2004).
J. Heyd, J. E. Peralta, G. E. Scuseria, and R. L. Martin, J. Chem. Phys., 123, Article ID 174101 (2005).
J. Heyd, G. E. Scuseria, and M. Ernzerhof, J. Chem. Phys., 124, Article ID 219906 (2006).
D. Avcı, S. Bahceli, O. Tamer, and Y. Atalay, Can. J. Chem., 93, 1147 (2015).
H. Pir Gumus, O. Tamer, D. Avcı, and Y. Atalay, J. Phys., 90, 1 (2015).
S. Sevvanthi, S. Muthu, S. Aayisha, P. Ramesh, and M. Raja, Chem. Data Coll., 30, Article ID 100574 (2020).
Author information
Authors and Affiliations
Corresponding author
Additional information
Abstract of article is published in Zhurnal Prikladnoi Spektroskopii, Vol. 89, No. 6, p. 897, November–December, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gümüş, H., Tekin, N. & Kara, Y.S. Structural and Spectroscopic Characterization of 3-[4-(Trifluoromethyl)Phenyl]-3a,4,8,8a-Tetrahydro-6H-[1,3] Dioxepino[5,6-d][1,2]Oxazole Compound: An Experimental and Density Functional Theory Study. J Appl Spectrosc 89, 1150–1157 (2023). https://doi.org/10.1007/s10812-023-01481-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-023-01481-2