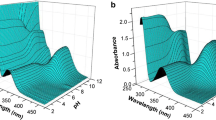
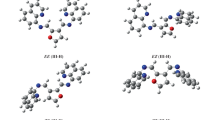
The π-conjugation pathways were identified and the degrees of aromaticity for NH-tautomers of corrole free bases were determined by quantum chemistry methods and absorption spectroscopy. The macrocycle skeletal atoms participated differently in the formation of the π-conjugation pathway. It was supposed that conjugation pathways consisting of 18 π-electrons were dominant. At the same time, each of two NH-tautomers possessed its own distinct π-conjugation pathway, which caused their degrees of aromaticity to differ. It was shown that the peripheral substitution architecture of the macrocycle influenced the degree of aromaticity. A method for controlling the equilibrium between the two NH-tautomers that consisted of designing the electron-density distribution in the macrocycle that was characteristic of one of the tautomers was proposed and proved experimentally.
Similar content being viewed by others
References
J. Juselius and D. Sundholm, Phys. Chem. Chem. Phys., 2, 2145–2151 (2000).
J.-I. Aichara, J. Phys. Chem. A, 112, 5305–5311 (2008).
T. D. Lash, J. Porphyrins Phthalocyanines, 15, 1093–1115 (2011).
H. Fliegl and D. Sundholm, J. Org. Chem., 77, 3408–3414 (2012).
T. M. Krygowski, H. Szatylowicz, O. A. Stasyuk, J. Dominikowska, and M. Palusiak, Chem. Rev., 114, 6383–6432 (2014).
T. Woller, P. Geerling, F. De Proft, B. Champagne, and M. Alonso, Molecules, 23, 1333 (2018).
D. B. Berezin, D. R. Karimov, and A. V. Kustov, Corroles and Their Derivatives: Synthesis, Properties, Prospects for Practical Application [in Russian], LENAND, Moscow (2018).
Yu. B. Ivanova, V. A. Savva, N. Zh. Mamardashvili, A. S. Starukhin, T. H. Ngo, W. Dehaen, W. Maes, and M. M. Kruk, J. Phys. Chem. A, 116, 10683–10694 (2012).
M. M. Kruk, T. H. Ngo, P. Verstappen, A. S. Starukhin, J. Hofkens, W. Dehaen, and W. Maes, J. Phys. Chem. A, 116, 10695–10703 (2012).
M. M. Kruk, T. H. Ngo, V. A. Savva, A. S. Starukhin, W. Dehaen, and W. Maes, J. Phys. Chem. A, 116, 10704–10711 (2012).
W. J. D. Beenken, M. Presselt, T. H. Ngo, W. Dehaen, W. Maes, and M. M. Kruk, J. Phys. Chem. A, 118, 862–871 (2014).
D. V. Petrova, A.S. Semeikin, N. M. Berezina, M. B. Berezin, and M. I. Bazanov, Macroheterocycles, 12, 119–128 (2019).
D. N. Laikov, Chem. Phys. Lett., 281, 151–156 (1997).
D. N. Laikov and Yu. A. Ustynyuk, Russ. Chem. Bull., 54, 820–826 (2005).
T. M. Krygowski, J. Chem. Inf. Comput. Sci., 33, 70–78 (1993).
N. N. Kruk, Structure and Optical Properties of Tetrapyrrole Compounds [in Russian], Minsk, BGTU (2019).
M. O. Senge, S. A. MacGowan, and J. O'Brien, Chem. Commun., 51, 17031–71063 (2015).
M. M. Kruk, D. V. Kelnitsky, and W. Maes, Macroheterocycles, 12, 58–67 (2019).
N. N. Kruk, D. V. Klenitsky, L. L. Gladkov, and W. Maes, Tr. BGTU, Ser. 3: Fiz.-Mat. Nauki Inf., 218, 20–26 (2019).
N. N. Kruk, D. V. Kelnitsky, and W. Maes, Tr. BGTU, Ser. 3 Fiz.-Mat. Nauki Inf., 230, 7–13 (2020).
Y. H. Ajeeb, D. V. Klenitsky, I. V. Vershilovskaya, D. V. Petrova, A. S. Semeikin, W. Maes, L. L. Gladkov, and M. M. Kruk, J. Appl. Spectrosc., 87, 421–427 (2020).
H. Fliegl, S. Taubert, O. Lehtonen, and D. Sundholm, Phys. Chem. Chem. Phys., 13, 202500–20518 (2011).
S. L. Murov, I. Carmichael, and G. L. Hug, Handbook of Photochemistry, 2nd edn., New York (1993).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 88, No. 6, pp. 836–844, November–December, 2021.
Rights and permissions
About this article
Cite this article
Klenitsky, D.V., Gladkov, L.L., Vershilovskaya, I.V. et al. Quantum-Chemical Calculation and Spectroscopic Study if π-Conjugation Pathway in NH-Tautomers of Corrole Free Bases. J Appl Spectrosc 88, 1111–1118 (2022). https://doi.org/10.1007/s10812-022-01287-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-022-01287-8