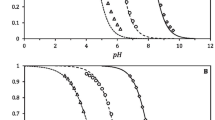
The rates of an enzymatic reaction are determined using spontaneous Raman spectroscopy. Time dependences of integrated Raman intensities in spectra of the reaction mixtures are analyzed. The rates of the α-chymotrypsincatalyzed reaction are determined at different temperatures and pH values.
Similar content being viewed by others
References
H. Bisswanger, Enzyme Kinetics: Principles and Methods, Wiley-VCH Verlag GmbH & Co., Germany (2017).
H. Bisswanger, Perspect. Sci., 1, 41–55 (2014).
D. L. Purich, Enzyme Kinetics: Catalysis & Control, Elsevier, Boston (2010).
H. U. Bergmeyer, Methods of Enzymatic Analysis, Academic Press, USA (1974).
J. Cielecka-Piontek, Appl. Spectrosc., 67, No. 7, 703–708 (2013).
F. Mirazizi, A. Bahrami, K. Haghbeen, H. Shahbani Zahiri, M. Bakavoli, and R. L. Legge, J. Enzyme Inhib. Med. Chem., 31, No. 6, 1162–1169 (2016).
B. Tang, F. Han, and L. Ma, Chin. J. Anal. Chem., 29, No. 3, 347–354 (2001).
U. Kettling, A. Koltermann, P. Schwille, and M. Eigen, Proc. Natl. Acad. Sci. USA, 95, No. 4, 1416–1420 (1998).
Y. C. Tsai, Z. Jin, and K. A. Johnson, Anal. Biochem., 384, No. 1, 136–144 (2009).
E. Navarro-Peran, J. Cabezas-Herrera, A. N. P. Hiner, T. Sadunishvili, F. Garcia-Canovas, and J. N. Rodriguez-Lopez, Biochemistry, 44, No. 20, 7512–7525 (2005).
T. K. Harris and M. M. Keshwani, Guide to Protein Purification, Methods in Enzymology, R. R. Burgess and M. P. Deutscher (Eds.), Vol. 463, Academic Press, USA (2009), pp. 57–71.
C. Aguirre, I. Condado-Morales, L. F. Olguin, and M. Costas, Anal. Biochem., 479, No. 15, 18–27 (2015).
M. Noda, Y. Matoba, T. Kumagai, and M. Sugiyama, Biochem. J., 389, 491–496 (2005).
F. Zsila, J. Phys. Chem. B, 117, No. 37, 10798–10806 (2013).
I. A. Balakhnina, N. N. Brandt, A. A. Mankova, A. Yu. Chikishev, and I. G. Shpachenko, J. Appl. Spectrosc., 84, No. 4, 650–656 (2017).
I. G. Shpachenko, N. N. Brandt, and A. Yu. Chikishev, Moscow Univ. Phys. Bull., 73, No. 6, 644–650 (2018).
N. Brun, I. Youssef, M.-C. Chevrel, D. Chapron, C. Schrauwen, S. Hoppe, P. Bourson, and A. Durand, J. Raman Spectrosc., 44, No. 6, 909–915 (2013).
I. Csontos, H. Pataki, A. Farkas, H. Bata, B. Vajna, Z. K. Nagy, G. Keglevich, and G. J. Marosi, Org. Process Res. Dev., 19, No. 1, 189–195 (2015).
D. Sahnic, E. Mestrovic, T. Jednacak, I. Habinovec, J. Parlov Vukovic, and P. Novak, Org. Process Res. Dev., 20, No. 12, 2092–2099 (2016).
M. Assirelli, W. Xu, and W. Chew, Org. Process Res. Dev., 15, No. 3, 610–621 (2011).
Y. Ou, R. E. Wilson, and S. G. Weber, Annu. Rev. Anal. Chem., 11, No. 1, 509–533 (2018).
I. A. Balakhnina, N. N. Brandt, A. Yu. Chikishev, A. A. Mankova, and I. G. Shpachenko, Vib. Spectrosc., 106, 103004 (2020).
F. J. Kezdy and M. L. Bender, Biochemistry, 1, No. 6, 1097–1106 (1962).
M. L. Bender, F. J. Kezdy, and F. C. Wedler, J. Chem. Educ., 44, No. 2, 84–88 (1967).
S. Z. Vatsadze, A. V. Medved'ko, S. A. Kurseev, O. I. Pokrovskiy, O. O. Parenago, M. O. Kostenko, I. V. Ananyev, K. A. Lyssenko, D. A. Lemenovsky, G. M. Kazankov, and V. V. Lunin, Organometallics, 36, 3068–3075 (2017).
S. Vatsadze, A. Medved'ko, G. Kazankov, and S. Kurzeev, ChemSpider Synthetic Pages, 705; http://cssp.chemspider.com.
N. N. Brandt and A. Yu. Chikishev, Laser Phys., 12, 647–652 (2002).
N. N. Brandt, A. Yu. Chikishev, A. I. Sotnikov, Yu. A. Savochkina, I. I. Agapov, and A. G. Tonevitsky, J. Mol. Struct., 735–736, 293–298 (2005).
N. N. Brandt, A. Yu. Chikishev, and V. N. Kruzhilin, Vib. Spectrosc., 89, 75–80 (2017).
N. N. Brandt, O. O. Brovko, A. Yu. Chikishev, and O. D. Paraschuk, Appl. Spectrosc., 60, 288–293 (2006).
H. J. Goren and M. Fridkin, Eur. J. Biochem., 41, No. 2, 263–272 (1974).
H. Gutfreund and J. M. Sturtevant, Biochem. J., 63, No. 4, 656–661 (1956).
K. K. Ghosh and S. K. Verma, Indian J. Biochem. Biophys., 45, No. 5, 350–353 (2008).
P. A. Adams and E. R. Swart, Biochem. J., 161, 83–92 (1977).
S. Verma, Indian J. Chem., Sect. A: Inorg., Bio-Inorg., Phys., Theor. Anal. Chem., 49, 1041–1046 (2010).
M. L. Bender, G. R. Schonbaum, and B. Zerner, J. Am. Chem. Soc., 84, No. 13, 2562–2570 (1962).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 88, No. 1, pp. 11–16, January–February, 2021.
Rights and permissions
About this article
Cite this article
Balakhnina, I.A., Brandt, N.N., Mankova, A.A. et al. Determination of α-Chymotrypsin-Catalyzed Reaction Rates at Different Temperatures and PH Values by Raman Spectroscopy. J Appl Spectrosc 88, 6–11 (2021). https://doi.org/10.1007/s10812-021-01133-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-021-01133-3