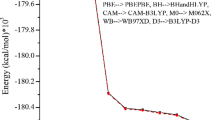
The optimized molecular structure, vibrational frequencies and corresponding vibrational assignments as well as 1H NMR and 13C NMR chemical shifts of 4-methyl anilinium phenolsulfonate have been investigated using quantum chemical calculations. Obtained results, in particular, on the geometric structure, chemical shifts of 1H and 13C, and vibrational frequencies are in good agreement with experimental data. The title compound was optimized using the BHandHLYP and WB97XD levels of density functional theory. The stability of the molecule arising from hyper-conjugative interaction and charge delocalization has been studied using natural bond orbital analysis. The HOMO and LUMO analysis is used to determine the charge transfer within the molecule. Polarizability and hyperpolarizability values are calculated at the same levels in order to find the importance of the title compound in nonlinear optics. The molecular electrostatic potential has been simulated to demonstrate electrophilic and nucleophilic sides of the title compound.
Similar content being viewed by others
References
N. B. Singh, T. Henningsen, E. P. A. Metz, R. Hamacher, E. Cumberledge, and R. H. Hopkins, Mater. Lett., 12, 270–275 (1991).
Nonlinear Optical Properties of Organic Molecules and Crystals, Eds. D. S. Chemla and J. Zyss, 1, Academic Press, London (1987).
J. Badan, R. Hierle, A. Perigaud, and J. Zuss, Am. Chem. Soc. Sympos. Ser., 233, 81–107 (1983).
R. Santhakumari, K. Ramamurthi, G. Vasuki, B.M. Yamin, and G. Bhagavannarayana, Spectrochim. Acta, A, 76, 369–375 (2010).
G. M. Frankenbach and M.C. Etter, J. Chem. Mater., 4, 272–278 (1992).
F. D. Proft and P. Geerlings, Chem. Rev., 101, 1451–1464 (2001).
Ö. Tamer, N. Dege, G. Demirtaş, D. Avcı, Y. Atalay, M. Macit, and S. Şahin, J. Mol. Struct., 1063, 295–306 (2014).
Ö. Tamer, D. Avcı, and Y. Atalay, Spectrochim. Acta, A, 117, 78–86 (2014).
A. Kunduracıoğlu, Ö. Tamer, D. Avcı, İ. Kani, Y. Atalay, and B. Çetinkaya, Spectrochim. Acta, A, 121, 35–45 (2014).
Ö. Tamer, D. Avcı, and Y. Atalay, J. Appl. Spectrosc., 80, 971–982 (2014).
H. Pir, N. Günay, Ö. Tamer, D. Avcı, and Y. Atalay, Spectrochim. Acta, A, 112, 331–342 (2013).
R. K. Balachandar, S. Kalainathan, and S. M. Eappen, J. Podder, Acta Crystallogr. E, 69, 905–1000 (2013).
G. Shanmugam and S. Brahadeeswaran, Spectrochim. Acta, A, 95, 177–183 (2012).
J. V. Jovita, K. Boopathi, P. Ramasamy, A. Ramanand, and P. Sagayaraj, J. Cryst. Growth, 380, 218–223 (2013).
Gaussian-09, Gaussian, Inc., Wallingford CT (2009).
M. J. Frisch, J. A. Pople, and J. S. Binkley, J. Chem. Phys., 80, 3265–3269 (1984).
GaussView, Version 5, Semichem Inc., Shawnee Mission KS (2009).
A. D. Becke, J. Chem. Phys., 98, 1372–1377 (1993).
J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 10, 6615–6620 (2008).
M. H. Jamróz, Vibrational Energy Distribition Analysis VEDA4, Warsaw, Poland (2004).
M. H. Jamróz and J.Cz. Dobrowolski, J. Mol. Struct., 565–566, 475–480 (2001).
R. E. Stratmann, G. E. Scuseria, and M. J. Frisch, J. Chem. Phys., 109, 8218–8284 (1998).
S. I. Gorelsky, SWizard Program Revision 4.5, University of Ottawa, Canada (2010) http://www.sg.chem.net.
R. Ditchfield, J. Chem. Phys., 56, 5688–5692 (1972).
A. Frish, A. B. Nielsen, and A. J. Holder, Gauss View User Manual, Gaussian Inc., Pittsburg, PA (2001)
W. H. James, E. G. Buchanan, C. W. Müller, J. C. Dean, D. Kosenkov, L. V. Slipchenko, L. Guo, A. G. Reidenbach, S. H. Gellman, and T. S. Zwier, J. Phys. Chem. A, 115, 13783–13798 (2011).
D. Shobaa, S. Periandy, M. Karabacak, and S. Ramalingam, Spectrochim. Acta, A, 83, 540–552 (2011).
F. R. Dollish, W. G. Fateley, and F. F. Bentley, Characteristic Raman Frequencies of Organic Compounds, John Wiley & Sons, New York (1997).
Ö. Tamer, N. Dege, G. Demirtaş, D. Avcı, Y. Atalay, M. Macit, and A. Alaman Ağar, Spectrochim. Acta, A, 117, 13–23 (2014).
V. Arjunan, M. K. Marchewka, A. Pietraszko, and M. Kalaivani, Spectrochim. Acta, A, 97, 625–638 (2012).
N. Sudharsana, G. Subramanian, V. Krishnakumar, and R. Nagalakshmi, Spectrochim. Acta, A, 97, 798–805 (2012).
H. Tanak, K. Pawlus, M. K. Marchewka, and A. Pietraszko, Spectrochim. Acta, A, 118, 82–93 (2014).
N. P. G. Roeges, A Guide to the Complete Interpretation of Infrared Spectra of Organic Structures, Wiley, New York (1994).
N. B. Colthup, L. H. Daly, and S. E. Wiberly, Introduction to Infrared and Raman Spectroscopy, 3rd ed., Academic Press, Boston (1990).
L. J. Bellamy, The Infrared Spectra of Complex Molecules, 3rd ed., Wiley, New York (1975).
A. Sher Gill and S. Kalainathan, J. Phys. Chem. Solids, 72, 1002–1007 (2011).
R. G. Pearson, Proc. Natl. Acad. Sci. USA, 83, 8440–8441 (1986).
H. Pir Gümüş, Ö. Tamer, D. Avcı, and Y. Atalay, Spectrochim. Acta, A, 129, 219–226 (2014).
H. Pir Gümüş, Ö. Tamer, D. Avcı, and Y. Atalay, Spectrochim. Acta, A, 132, 183–190 (2014).
R. S. Mulliken, J. Chem. Phys., 23, 1833–1840 (1955).
J. S. Murray and K. Sen, Molecular Electrostatic Potentials, Concepts and Applications, Elsevier, Amsterdam (1996).
E. Scrocco and J. Tomasi, Adv. Quantum Chem., 11, 115–121 (1978).
A. E. Reed, F. Weinhold, R. Weiss, and J. Macheleid, J. Phys. Chem., 89, 2688–2694 (1985).
N. Öner, Ö. Tamer, D. Avcı, and Y. Atalay, Spectrochim. Acta, A, 133, 542–549 (2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Abstract of article is published in Zhurnal Prikladnoi Spektroskopii, Vol. 82, No. 4, p. 645, July–August, 2015.
Rights and permissions
About this article
Cite this article
Tamer, Ö., Avcı, D. & Atalay, Y. Geometry Optimization, Spectral Analysis, Molecular Electrostatic Potential Surface, and Nonlinear Optical Activity of 4-Methyl Anilinium Phenolsulfonate: a DFT Study. J Appl Spectrosc 82, 687–699 (2015). https://doi.org/10.1007/s10812-015-0165-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-015-0165-1