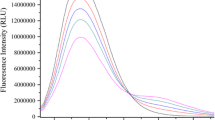
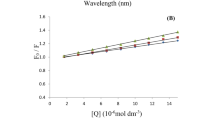
The interaction of nebivolol hydrochloride (NH), a β1-blocker, with bovine serum albumin (BSA) has been investigated at different pH values using the fluorescence quenching technique. The effect of different temperatures was studied at physiological pH 7.4. The binding constants of NH with BSA at 288, 298, and 309 K were found to be 2.691 × 1011, 1.38 × 1010, and 6.27 × 108 M−1, respectively. From the Arrhenius plot, the thermodynamic parameters, ΔH0 and ΔS0, were estimated to be –204.48 kJ/mol and –491.42 J/mol × K, respectively. This indicates that Van der Waals interactions and hydrogen bonds play a major role in the reaction. The effect of some inorganic divalent cations (Cu2+, Ni2+, and Zn2+) on binding of NH to BSA was also studied at physiological pH 7.4. Conformational investigation of BSA was done using synchronous fluorescence, showing the change in the microenvironment of the tryptophan residues. Fluorescence quenching reactions of NH to human serum albumin (HSA) and to γ-globulins were investigated and the binding parameters were obtained.
Similar content being viewed by others
References
K. Yamasaki, T. Maruyama, U. Kragh-Hansen, and M. Otagiri, Biochim. Biophys. Acta, 1295, 147–157 (1996).
B. P. Kamat and J. Seetharamappa, J. Pharm. Biomed. Anal., 35, 655–664 (2004).
M. D. Reed, C. M. Myers, and J. L. Blumer, Curr. Ther. Res., 8, 558–565 (2001).
N. Seedher, Ind. J. Pharm. Sci., 62, 16–20 (2000).
D. Silva, C. M. Cortez, J. Cunha-Bastos, and S. R. W. Louro, Toxicol. Lett., 147, 53–61 (2004).
A. Rieutord, P. Bourget, G. Torche, and J. F. Zazzo, Int. J. Pharm., 119, 57–62 (1995).
O. Borga and B. Borga, J. Pharmacokinet. Biopharm., 25, 63–77 (1997).
B. Klajnert, L. Stanisławska, M. Bryszewska, and B. Pałecz, Biochim. Biophys. Acta, 1648, 115–126 (2003).
T. J. Peters, All About Albumin. Biochemistry, Genetics, and Medical Applications, Academic Press, San Diego (1996).
Y. Xu, H. X. Shen, and H. G. Huang, Chem. J. Chin. Univ., 17, 1855–1858 (1996).
K. Yamasaki, T. Maruyama, K. Yoshimoto, Y. Tsutsumi, R. Narazaki, A. Fukuhara, U. Kragh-Hansen, and M. Otagiri, Biochim. Biophys. Acta, 1432, 313–323 (1999).
J. Wilting, W. F. van Der Giesen, L. H. M. Janssen, M. M. Weideman, M. Otagiri, and J. H. Perrin, J. Biol. Chem., 255, 3032–3037 (1980).
Y. Q. Wang, H. M. Zhang, G. C. Zhang, J. Pharm. Biomed. Anal., 41, 1041–1046 (2006).
P. B. Kandagal, S. Ashoka, J. Seetharamappa, S. M. T. Shaikh, Y. Jadegoud, and O. B. Ijare, J. Pharm. Biomed. Anal., 41, 393–399 (2006).
S. Baroni, M. Mattu, A. Vannini, R. Cipollone, S. Aime, P. Ascenzi, and M. Fasano, Eur. J. Biochem., 268, 6214–6220 (2001).
E. Karnaukhova, Biochem. Pharmacol., 73, 901–910 (2007).
C. D. Kanakis, P. A. Tarantilis, M. G. Polissiou, S. Diamantoglou, and H. A. Tajmir-Riahi, J. Mol. Struct., 798, 69–74 (2006).
H. Gao, L. D. Lei, J. Q. Liu, Q. Kong, X. G. Chen, and Z. D. Hu, J. Photochem. Photobiol. A, 167, 213–221 (2004).
P. B. Kandagal, S. M. T. Shaikh, D. H. Manjunatha, J. Seetharamappa, and B. S. Nagaralli, J. Photochem. Photobiol. A, 189, 121–127 (2008) .
A. Sułkowska, B. Bojko, J. Rownicka, P. Rezner, and W. W. Sułkowski, J. Mol. Struct., 744, 781–787 (2006).
M. Hepel, Electroanalysis, 17, 1401–1412 (2005).
Q. Zhang, S. Ni, and Y. Kokot, Talanta, 88, 524–532 (2012).
I. Girard and S. Ferry, J. Pharm. Biomed., 14, 583–591 (1996).
M. C. Millot, S. Servagent-Noinville, N. L. Taleb, M. H. Baron, M. Revault, and B. Sebille, J. Chromatogr. B: Biomed. Sci. Appl., 753, 101–113 (2001).
Y. Li, W. Y. He, H. X. Liu, X. J. Yao, and Z. D. Hu, J. Mol. Struct., 831, 144–150 (2007).
J. Oravcova, B. Bobs, and W. Lindner, J. Chromatogr. B, 677, 1–28 (1996).
D. E. Epps, T. J. Raub., V. Caiolfa, A. Chiari, and M. Zamai, J. Pharm. Pharmacol., 51, 41–48 (1998).
M. R. Bristow, P. Nelson, W. Minobe, and C. Johnson, Am. J. Hypertension, 18, N 5, Supp A51–A52 (2005).
A. C. Moffat, M. D. Osselton, and B. Widdop, Clarke’s Analysis of Drugs and Poisons, 3rd ed., 2 (2004).
S. Ashoka, J. Seetharamappa, P. Kandagal, and S. Shaikh, J. Lumin., 121, N 1, 179–186 (2006).
B. Liu, C. Yang, X. Yan, J. Wang, and L. Yunkai, Int. J. Anal. Chem., 2012 (2012); doi:10.1155/2012/408057.
J. R. Lakowicz, Principles of Fluorescence Spectroscopy, 3rd ed., Springer, New York (2006).
Y. J. Hu, Y. Liu, J. B. Wang, X. H. Xiao, and S. S. Qu, J. Pharm. Biomed. Anal., 36, 915–919 (2004).
N. Subhan, R. Habibur, A. Ashraful, I. Rashedul, and R. Mahbubur, Rom. J. Biophys. 21, No. 2, 139–149 (2011).
J. Sereikaite, Z. Bumeliene, and V. A. Bumelis, Acta Chromatogr., 15, 298–306 (2005).
M. Dockal, D. C. Carter, and F. Ruker, J. Biol. Chem., 275, 3042–3050 (2000).
V. M. Rosenoer, M. Oratz, and M. A. Rothschild, Albumin. Structure, Function, and Uses, Pergamon Press, New York (1977).
A. Sukowska, J. Mol. Struct., 614, No. 1–3, 227–232 (2002).
L. Trynda-Lemiesz, B. Keppler, and H. Koztowski, J. Inorg. Biochem., 73, 123–128 (1999).
M. R. Eftink and C. A. Ghiron, J. Phys. Chem., 80, 486–493 (1976).
N. Zhou, Y. Liang, and P. Wang, J. Mol. Struct., 872, No. 2–3, 190–196 (2008).
F. Rasoulzadeh, D. Asgari, A. Naseri, and M. R. Rashidi, DARU, 18, No. 3, 179–184 (2010).
Y. Wang, H. Zhang, G. Zhang, W. Tao, and S. Tang, J. Mol. Struct., 830, 40–45 (2007)
F. Bogdan, A. Pirnau, C. Floare, and C. Bugeac, J. Pharm. Biomed. Anal., 47, 981–984 (2008).
W. R. Ware, J. Phys. Chem., 66, 455–458 (1962).
G. Neméthy and H. Scheraga, J. Phys. Chem., 66, 1773–1789 (1962).
S. Timasheff, Proteins of Biological Fluids, Ed. H. Peeters, Pergamon Press, Oxford (1972).
P. Ross and S. Subramanian, Biochemistry, 20, 3096–3102 (1981).
Y. J. Hua, Y. Liu, L. X. Zhang, and R. M. Zhao, J. Mol. Struct., 750, 174–178 (2005).
M. Rahman, T. Maruyama, T. Okada, K. Yamasaki, and M. Otagiri, Biochem. Pharmacol., 46, 1721–1731 (1993).
J. Zhao, X. Jiang, X. Liu, and F. Ren, Arch. Biol. Sci. Belgrade, 63, No. 2, 325–331 (2011).
J. Jayabharathi, V. Thanikachalam, and M. V. Perumal, J. Lumin., 132, 707–712 (2012).
Y. Zhang, S. Shi, K. Huang, X. Chen, and M. Peng, J. Lumin., 131, 1927–1931 (2011)
R. G. Machicote, M. E. Pacheco, and L. Bruzzone, Spectrochim. Acta, A, 77, 466–472 (2010)
G. Z. Chen, X. Z. Huang, J. G. Xu, Z. Z. Zheng, and Z. B. Wang, The Methods of Fluorescence Analysis, 2nd ed., Beijing Science Press (1990).
J. N. Miller, Anal. Proc., 16, 203–209 (1979)
K. H. Ulrich, Pharmacol. Rev., 33, 17–53 (1981).
F. Meng, J. Zhu, A. Zhao, S. Yu, and C. Lin, J. Lumin., 132, 1290–1298 (2012).
F. Cui, Y. Yan, Q. Zhang, X. Yao, G. Qu, and Y. Lu , Spectrochim. Acta, A, 74, 964–971 (2009).
X. Pan, R. Liu, P. Qin, L. Wang, and X. Zhao, J. Lumin., 130, 611–617 (2010).
G. Zhang, N. Zhao, and L.Wang, J. Lumin., 131, 880–887 (2011).
I. Petitpas, T. Grune, A. A. Bhattacharya, and S. Curry, J. Mol. Biol., 314, 955–960 (2001).
G. D. Olsen, Clin. Pharmacol. Ther., 17, No. 1, 31–35 (1975).
H. Sun, M. S. Chow, and E. G. Maderazo, Antimicrob. Agents Chemother., 35, 112232–112237 (1991).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Zhurnal Prikladnoi Spektroskopii, Vol. 82, No. 4, pp. 584–591, July–August, 2015.
Rights and permissions
About this article
Cite this article
Abdel-Aziz, L., Abdel-Fattah, L., El-Kosasy, A. et al. A Fluorescence Quenching Study of the Interaction of Nebivolol Hydrochloride with Bovine and Human Serum Albumin. J Appl Spectrosc 82, 620–627 (2015). https://doi.org/10.1007/s10812-015-0154-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-015-0154-4