Abstract
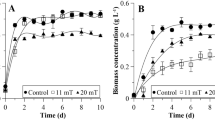
The main objective of this research was to evaluate the effect of a 47 mT static magnetic field (SMF) applied at different times of the exponential growth phase of a Chlorella microalgae-bacteria consortium. For this, growth parameters, cell division, the photochemical activity of photosystem II (PS II), and the biochemical composition of the microorganisms were studied. Biomass concentration and productivity of cultures exposed to SMF increased concerning control cultures, reaching maximum values when this physical agent was applied in the early exponential phase, 0.89 g L−1 and 0.075 g L−1 d−1, respectively. In addition, SMF application stimulated binary and multiple cell division of cultures exposed during early and late exponential phases. PS II quantum yield was significantly increased over control cultures immediately after applying SMF during early (0.70) and late (0.73) exponential phases. In addition, in cultures exposed to SMF, the quantum yield for electron transport (ϕEo) increased, and the absorption flux per reaction center (ABS/RC) decreased, which was associated with an increase in the active reaction centers of PS II. Extracellular protein, carbohydrate, and polysaccharide content varied when SMF was applied during the early exponential phase. No significant differences were observed regarding the lipid content of the control cultures and those exposed to SMF. It is concluded that SMF increases the formation of radical pairs in photosystem II due to the increase in the number of active reaction centers, which could constitute the mechanism of action of this system.












Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Barber J (2003) Photosystem II: The engine of life. Quart Rev Biophys 36:71–89
Bauer LM, Costa JAV, da Rosa APC, Santos LO (2017) Growth stimulation and synthesis of lipids, pigments and antioxidants with magnetic fields in Chlorella kessleri cultivations. Bioresour Technol 244:1425–1432
Bellou S, Baeshen MN, Elazzazy AM, Aggeli D, Sayegh F, Aggelis G (2014) Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol Adv 32:1476–1493
Bišová K, Zachleder V (2014) Cell-cycle regulation in green algae dividing by multiple fission. J Exp Bot 65:2585–2602
Björn LO, Papageorgiou GC, Blankenship RE (2009) A viewpoint: why chlorophyll a? Photosynth Res 99:85–98
Borowitzka MA (2016) Algal physiology and large-scale outdoor cultures of microalgae. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 601–652
Brailo M, Pećarević M, Grilec D, Mišković M, Lale D, Jurjević M, Čalić M, Mikuš J, BratošCetinić A (2018) The influence of electromagnetic field on viability of marine microalgae Tetraselmis suecica and bacteria Escherichia coli and Enterococcus faecalis. Naše More 65:71–77
Chu F-J, Wan T-J, Pai T-Y, Lin H-W, Liu S-H, Huang C-F (2020) Use of magnetic fields and nitrate concentration to optimize the growth and lipid yield of Nannochloropsis oculata. J Environ Manage 253:109680
Costa SS, Peres BP, Machado BR, Costa JAV, Santos LO (2020) Increased lipid synthesis in the culture of Chlorella homosphaera with magnetic fields application. Bioresour Technol 315:123880
Croce R, van Amerongen H (2020) Light harvesting in oxygenic photosynthesis: Structural biology meets spectroscopy. Science 369:eaay2058
Dao LH, Beardall J (2016) Effects of lead on two green microalgae Chlorella and Scenedesmus: Photosystem II activity and heterogeneity. Algal Res 16:150–159
Deamici KM, Cardias BB, Costa JAV, Santos LO (2016) Static magnetic fields in culture of Chlorella fusca: Bioeffects on growth and biomass composition. Process Biochem 51:912–916
Deamici KM, Cuellar-Bermudez SP, Muylaert K, Santos LO, Costa JAV (2019a) Quantum yield alterations due to the static magnetic fields action on Arthrospira platensis SAG 21.99: Evaluation of photosystem activity. Bioresour Technol 292:121945
Deamici KM, Santos LO, Costa JAV (2019b) Use of static magnetic fields to increase CO2 biofixation by the microalga Chlorella fusca. Bioresour Technol 276:103–109
Deamici KM, Santos LO, Costa JAV (2021) Magnetic field as promoter of growth in outdoor and indoor assays of Chlorella fusca. Bioprocess Biosyst Eng 44:1453–1460
Du N, Gholami P, Kline DI, DuPont CL, Dickson AG, Mendola D, Martz T, Allen AE, Mitchell BG (2018) Simultaneous quantum yield measurements of carbon uptake and oxygen evolution in microalgal cultures. PloS One 13:e0199125
Eiler A, Olsson JA, Bertilsson S (2006) Diurnal variations in the auto-and heterotrophic activity of cyanobacterial phycospheres (Gloeotrichia echinulata) and the identity of attached bacteria. Freshw Biol 51:298–311
Font YS, Díaz YO, Cuypers A, Alemán EI, Vandamme D (2023) The effect of magnetic field treatment on the cultivation of microalgae: An overview of involved mechanisms. J Appl Phycol 35:1525–1536
Gateau H, Solymosi K, Marchand J, Schoefs B (2017) Carotenoids of microalgae used in food industry and medicine. Mini Rev Med Chem 17:1140–1172
Gautam H, Sharma A, Trivedi PK (2023) Plant microProteins and miPEPs: Small molecules with much bigger roles. Plant Sci 326:111519
Gonçalves AL, Pires JC, Simões M (2016) Wastewater polishing by consortia of Chlorella vulgaris and activated sludge native bacteria. J Clean Prod 133:348–357
Han S, Jin W, Chen Y, Tu R, Abomohra AE-F (2016) Enhancement of lipid production of Chlorella pyrenoidosa cultivated in municipal wastewater by magnetic treatment. Appl Biochem Biotech 180:1043–1055
Hess SK, Lepetit B, Kroth PG, Mecking S (2018) Production of chemicals from microalgae lipids–status and perspectives. Eur J Lipid Sci Technol 120:1700152
Ho TY, Quigg A, Finkel ZV, Milligan AJ, Wyman K, Falkowski PG, Morel FM (2003) The elemental composition of some marine phytoplankton. J Phycol 39:1145–1159
Hore PJ, Mouritsen H (2016) The radical-pair mechanism of magnetoreception. Annu Rev Biophys 45:299–344
Iqbal K, Saxena A, Pande P, Tiwari A, Joshi NC, Varma A, Mishra A (2022) Microalgae-bacterial granular consortium: Striding towards sustainable production of biohydrogen coupled with wastewater treatment. Bioresour Technol 354:127203
Ji X, Li H, Zhang J, Saiyin H, Zheng Z (2019) The collaborative effect of Chlorella vulgaris-Bacillus licheniformis consortia on the treatment of municipal water. J Hazard Mater 365:483–493
Junge W (2019) Oxygenic photosynthesis: history, status and perspective. Quart Rev Biophys 52:e1
Khorshidi N, Hassanpour H, Ziyadi H (2022) Static magnetic field improved growth and astaxanthin production in Haematococcus lacustris via the regulation of carbohydrate accumulation, H2O2 level, and antioxidant defense system. J Appl Phycol 34:2283–2295
Konopacki M, Rakoczy R (2019) The analysis of rotating magnetic field as a trigger of gram-positive and gram-negative bacteria growth. Biochem Eng J 141:259–267
Kula M, Kalaji H, Skoczowski A (2017) Culture density influence on the photosynthetic efficiency of microalgae growing under different spectral compositions of light. J Photochem Photobiol B 167:290–298
Leister D (2022) Enhancing the light reactions of photosynthesis: Strategies, controversies and perspectives. Mol Plant 16:4–22
Levasseur W, Perré P, Pozzobon V (2020) A review of high value-added molecules production by microalgae in light of the classification. Biotechnol Adv 41:107545
Li K, Liu Q, Fang F, Luo R, Lu Q, Zhou W, Huo S, Cheng P, Liu J, Addy M (2019a) Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour Technol 291:121934
Li N, Liu Z, Wang P, Suman K, Zhang J, Song Y (2022) Effects of sodium hypochlorite treatment on the chlorophyll fluorescence in photosystem II of microalgae. Sci Total Environ 833:155192
Li X, Li W, Zhai J, Wei H, Wang Q (2019b) Effect of ammonium nitrogen on microalgal growth, biochemical composition and photosynthetic performance in mixotrophic cultivation. Bioresour Technol 273:368–376
Luo X, Zhang H, Li Q, Zhang J (2020) Effects of static magnetic field on Chlorella vulgaris: growth and extracellular polysaccharide (EPS) production. J Appl Phycol 32:2819–2828
Luo X, Zhang H, Zhang J (2021) The influence of a static magnetic field on a Chlorella vulgaris-Bacillus licheniformis consortium and its sewage treatment effect. J Environ Manage 295:112969
Mamedov M, Nadtochenko V, Semenov A (2015) Primary electron transfer processes in photosynthetic reaction centers from oxygenic organisms. Photosynth Res 125:51–63
Markou G, Dao LH, Muylaert K, Beardall J (2017) Influence of different degrees of N limitation on photosystem II performance and heterogeneity of Chlorella vulgaris. Algal Res 26:84–92
Masojıdek J, Koblızek M, Torzillo G (2004) Photosynthesis in microalgae. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell Science, Oxford, pp 20–39
Melkozernov AN, Schmid VH, Schmidt GW, Blankenship RE (1998) Energy redistribution in heterodimeric light-harvesting complex LHCI-730 of photosystem I. J Phys Chem B 102:8183–8189
Moheimani NR, Borowitzka MA, Isdepsky A, Fon Sing S (2013) Standard methods for measuring growth of algae and their composition. In: Borowitzka MA, Moheimani NR (eds) Algae for Biofuels and Energy. Springer, Dordrecht, pp 265–284
Palmer CM (1959) Algae in water supplies: an illustrated manual on the identification, significance, and control of algae in water supplies, vol 2. US Department of Health, Education and Welfare, Public Health Service, Cincinnati
Pareek S, Sagar NA, Sharma S, Kumar V, Agarwal T, González‐Aguilar GA, Yahia EM (2017) Chlorophylls: Chemistry and biological functions. In: Yahia EM (ed) Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd Edn. Wiley-Blackwell, Hoboken pp 269–284
Plyusnina TY, Khruschev S, Degtereva N, Konyukhov I, Solovchenko A, Kouzmanova M, Goltsev V, Riznichenko G, Rubin A (2020) Gradual changes in the photosynthetic apparatus triggered by nitrogen depletion during microalgae cultivation in photobioreactor. Photosynthetica 58:443–451
Raouia H, Hamida B, Khadidja A, Ahmed L, Abdelwaheb C (2020) Effect of static magnetic field (200 mT) on biofilm formation in Pseudomonas aeruginosa. Arch Microbiol 202:77–83
Ritchie RJ (2008) Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 46:115–126
Rossi F, De Philippis R (2016) Exocellular polysaccharides in microalgae and cyanobacteria: Chemical features, role and enzymes and genes involved in their biosynthesis. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 565–590
Sajjadi B, Chen W-Y, Raman AAA, Ibrahim S (2018) Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew Sustain Energy Rev 97:200–232
Santos LO, Deamici KM, Menestrino BC, Garda-Buffon J, Costa JAV (2017) Magnetic treatment of microalgae for enhanced product formation. World J Microbiol Biotechnol 33:169
Shao W, Ebaid R, Abomohra AE-F, Shahen M (2018) Enhancement of Spirulina biomass production and cadmium biosorption using combined static magnetic field. Bioresour Technol 265:163–169
Shen J-R (2015) The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Annu Rev Plant Biol 66:23–48
Show PL (2022) Global market and economic analysis of microalgae technology: Status and perspectives. Bioresour Technol 357:127329
Silveira-Font Y, Gómez-Luna L, Kufundala-Wemba MD, Salazar-Hernández D, Ortega-Díaz Y (2018) Variación de la composición de pigmentos de Chlorella vulgaris Beijerinck, con la aplicación del campo magnético estático. Rev Cubana Quím 30:55–67
Singh H, Kumar D, Soni V (2022) Performance of chlorophyll a fluorescence parameters in Lemna minor under heavy metal stress induced by various concentration of copper. Sci Rep 12:10620
Siqueira SF, Queiroz MI, Zepka LQ, Jacob-Lopes E (2018) Introductory chapter: Microalgae biotechnology. A brief introduction. In: Jacob-Lopes E, Zepka LQ, Queiroz MI (eds) Microalgal Biotechnology. IntechOpen, Riejeka pp 1–11
Small DP, Hüner NP, Wan W (2012) Effect of static magnetic fields on the growth, photosynthesis and ultrastructure of Chlorella kessleri microalgae. Bioelectromagnetics 33:298–308
Solimeno A, García J (2017) Microalgae-bacteria models evolution: From microalgae steady-state to integrated microalgae-bacteria wastewater treatment models–a comparative review. Sci Total Environ 607:1136–1150
Solovchenko A, Solovchenko O, Khozin-Goldberg I, Didi-Cohen S, Pal D, Cohen Z, Boussiba S (2013) Probing the effects of high-light stress on pigment and lipid metabolism in nitrogen-starving microalgae by measuring chlorophyll fluorescence transients: Studies with a Δ5 desaturase mutant of Parietochloris incisa (Chlorophyta, Trebouxiophyceae). Algal Res 2:175–182
Stirbet A (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basics and applications of the OJIP fluorescence transient. J Photochem Photobiol B 104:236–257
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence. Springer, Dordrecht, pp 321–362
Su Y, Song K, Zhang P, Su Y, Cheng J, Chen X (2017) Progress of microalgae biofuel’s commercialization. Renew Sustain Energy Rev 74:402–411
Suresh Kumar A, Mody K, Jha B (2007) Bacterial exopolysaccharides–a perception. J Basic Microbiol 47:103–117
Veiga MC, Fontoura MM, de Oliveira MG, Costa JAV, Santos LO (2020) Magnetic fields: Biomass potential of Spirulina sp. for food supplement. Bioprocess Biosyst Eng 43:1231–1240
Vinyard DJ, Ananyev GM, Charles Dismukes G (2013) Photosystem II: The reaction center of oxygenic photosynthesis. Annu Rev Biochem 82:577–606
Wang Y, Ning W, Han M, Gao C, Guo W, Chang J-S, Ho S-H (2023) Algae-mediated bioremediation of ciprofloxacin through a symbiotic microalgae-bacteria consortium. Algal Res 71:103062
Wong SY, Wei Y, Mouritsen H, Solov’yov IA, Hore P (2021) Cryptochrome magnetoreception: Four tryptophans could be better than three. J R Soc Interface 18:20210601
Yan N, Fan C, Chen Y, Hu Z (2016) The potential for microalgae as bioreactors to produce pharmaceuticals. Int J Mol Sci 17:962
Yao S, Lyu S, An Y, Lu J, Gjermansen C, Schramm A (2019) Microalgae–bacteria symbiosis in microalgal growth and biofuel production: a review. J Appl Microbiol 126:359–368
Zachleder V, Bišová K, Vítová M (2016) The cell cycle of microalgae. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 3–46
Zachleder V, Ivanov IN, Kselíková V, Bialevich V, Vítová M, Ota S, Takeshita T, Kawano S, Bišová K (2021) Characterization of growth and cell cycle events affected by light intensity in the green alga Parachlorella kessleri: A new model for cell cycle research. Biomolecules 11:891
Zhao B, Sha H, Li J, Cao S, Wang G, Yang Y (2020) Static magnetic field enhanced methane production via stimulating the growth and composition of microbial community. J Clean Prod 271:122664
Zieliński M, Dębowski M, Kazimierowicz J (2021a) The effect of static magnetic field on methanogenesis in the anaerobic digestion of municipal sewage sludge. Energies 14:590
Zieliński M, Zielińska M, Cydzik-Kwiatkowska A, Rusanowska P, Dębowski M (2021b) Effect of static magnetic field on microbial community during anaerobic digestion. Bioresour Technol 323:124600
Funding
The authors are thankful for the financial support from Project 1 and Project 5 of the Programme VLIR-IUC-UOS – Universidad de Oriente CU2019IUC030A105-77143 and the UHasselt BOF-BILA fellowship R-9903.
Author information
Authors and Affiliations
Contributions
Y.S. Font: Conceptualization, Investigation, validation, visualization, writing-original Draft Y.O. Díaz: Conceptualization, writing – Review and editing A. Cuypers: Conceptualization, Resources, Writing – Review and Editing, funding acquisition, E.I Alemán: writing – review and editing, validation, funding acquisition, supervision; D. Vandamme: conceptualization, resources, writing – Review and editing – supervision – funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silveira Font, Y., Ortega Díaz, Y., Cuypers, A. et al. Influence of a static magnetic field on the photosynthetic apparatus, cell division, and biomass composition of a Chlorella microalgae-bacteria consortium. J Appl Phycol 36, 41–56 (2024). https://doi.org/10.1007/s10811-023-03137-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03137-2