Abstract
The biodiversity benefits of kelp aquaculture and afforestation are increasingly acclaimed as the industry continues to grow and develop globally, however, whether farmed kelp can provide this ecosystem service remains unclear. Using peer-reviewed literature, we evaluated whether kelp farms provide biodiversity benefits, and identified only 23 studies that discussed the effects of kelp aquaculture on biodiversity, half of which were broad reviews that only assessed the concept of ‘biodiversity’ peripherally (e.g. did not focus on specific responses or taxa). There is also a general lack of experimental research on the topic. Based on the evidence, it seems that kelp farms can create habitat via changes to the local environment, particularly through the provision of structure and changed nutrient cycling. While this can lead to increased abundance and diversity among certain taxa (e.g. fouling organisms), it seems that kelp farms typically create novel habitats that support distinct communities not equivalent to natural kelp forests. Moreover, the potential for kelp farms to support biodiversity depends on a range of operational factors, many of which may be at odds with farming objectives that require the harvest and removal of the habitat that farms provide. While more work needs to be done to address the complexity of comparisons between kelp farms and forests, especially at appropriate experimental scales, it currently seems unlikely that kelp farms will act as kelp forests and deliver meaningful biodiversity outcomes. We should instead recognise farms for providing their own valuable services and support restoration and conservation practices of kelp forests to pursue biodiversity outcomes.
Similar content being viewed by others
Introduction
Global growth in aquaculture has been increasingly met by a demand for ecological sustainability and a growing recognition of the environmental benefits that the industry may be able to support (Buschmann et al. 2017; Kim et al. 2017; Gentry et al. 2020). With the need to minimise or avoid negative environmental impacts, the ability of some aquaculture operations to deliver an environmentally sustainable product and provide ecosystem services (Hasselström et al. 2018; Theuerkauf et al. 2021; Barrett et al. 2022) will be an important component of the future development of the industry.
This is especially true for seaweed aquaculture, which has grown rapidly in both scale and interest over recent years. Globally, seaweed aquaculture produces ~30 million tonnes of farmed seaweeds annually (FAO 2020), with an estimated value of USD$13.7 billion (inflation adjusted to 2022; FAO 2018). As an extractive crop with little need for fertilisers or irrigation (Hasselström et al. 2018; Langton et al. 2019) the environmental impacts of seaweed aquaculture are likely more benign than those of other forms of fertilised agriculture and fed aquaculture (Zhang et al. 2009; Walls et al. 2017b; Visch et al. 2020). Seaweed farming has also been suggested to provide services including carbon sequestration, nutrient uptake, and habitat provisioning (Buschmann et al. 2017; Hasselström et al. 2018; Gentry et al. 2020), and in some circles has been lauded as a ‘silver bullet’ solution to climate change, coastal degradation, and food security (discussed by Grebe et al. 2019; Gentry et al. 2020; Costa-Pierce and Chopin 2021). This has also led to increased focus on afforestation practices which aim to farm seaweeds in novel areas and environments where it previously did not occur (e.g. kelp in the open ocean) for ‘ecosystem regeneration’ and biomass production (Bach et al. 2021; Boyd et al. 2022).
Evidence suggests that seaweed farms could support a number of valuable ecosystem services, such as nutrient cycling and absorption (e.g. Hasselström et al. 2018; Gentry et al. 2020). Nonetheless, the provision of many other potential ecosystem services by seaweed farms remains understudied or unclear (Hasselström et al. 2018; Gentry et al. 2020; Bach et al. 2021). One consequence is that ‘hype’ could lead to overpromising that hinders industry development and research and causes loss of social license if expectations are not met (Costa-Pierce and Chopin 2021). Indeed, it is becoming evident that there is more complexity to the provisioning of many of these ecosystem services than widely recognised (e.g. ecosystem exports/subsidies and biogeochemical processes affecting nutrient and carbon cycling and sequestration [Bach et al. 2021; Gallagher et al. 2022; Wright et al. 2022]), and that there may be important trade-offs to consider (discussed by Hasselström et al. 2018; Gentry et al. 2020; Barrett et al. 2022).
In light of these complexities, one of the most pressing needs is for an evaluation of the benefits to biodiversity from kelp aquaculture and afforestation. Biodiversity benefits can constitute enhanced species richness, abundance, biomass, and functional diversity (e.g. diversity in ecological function/role, morphology, and behavioural traits), and such benefits can also extend to the services that biodiversity supports and enhances, including fisheries production, recreation, and cultural services (Bennett et al. 2015; Krumhansl et al. 2016; Eger et al. 2021). Certainly, the biodiversity benefits of natural kelp forests are well understood, and kelps create complex habitats and serve as the trophic and physical foundation for productive and biodiverse ecosystems (Smale et al. 2013; Steneck and Johnson 2014). Like their natural counterparts, farmed kelp potentially also creates habitat that supports communities of associated organisms by providing structure, attachment sites, and trophic subsidies (Hasselström et al. 2018; Visch et al. 2020; Theuerkauf et al. 2021). If so, kelp farms may provide similarly valuable services that could contribute to restoration, conservation, or socioeconomic outcomes (discussed by Gentry et al. 2020; Layton et al. 2020), as may be the case for some other forms of aquaculture (e.g. shellfish; Theuerkauf et al. 2021; Barrett et al. 2022). However, important theoretical and practical issues remain, as there is a growing recognition that any biodiversity benefits of kelp farms may be minor or highly variable (e.g. Zhou 2012; Hehre and Meeuwig 2015; Walls et al. 2017b), and also challenging to achieve in a commercial setting (Buschmann et al. 2017; Wood et al. 2017; Campbell et al. 2019).
Here we review the available literature to assess the current evidence for kelp aquaculture and afforestation to provide meaningful biodiversity benefits (i.e. enhanced species richness, abundance, biomass, and functional diversity). Doing so may help avoid unfounded ‘hype’ and aid in preserving the scientific trust and social license that will be essential to the sustainable growth and development of the industry (Bax et al. 2022; Costa-Pierce and Chopin 2021). Evaluating whether kelp aquaculture and infrastructure can provide these valuable ecosystem services is one critical part of understanding its potential to aid in the market-driven restoration and conservation of marine ecosystems around the globe (also see Filbee-Dexter et al. 2022).
Methods
We first attempted a systematic review of peer-reviewed literature focussed on any effects of kelp aquaculture or afforestation on biodiversity. This yielded a very limited number of studies (detailed below), none of which focussed specifically on afforestation. Consequently, that small pool of results was supplemented by a broader search in efforts to utilise a wider range of literature and topics (e.g. community ecology, habitat structure, hydrodynamics) and offer new insights on past works.
For the initial systematic search, we used the following search terms in Scopus and Web of Science in October 2021 ([kelp OR seaweed OR macroalga*] AND [aquaculture OR farm* OR mariculture OR afforest*] AND [habitat OR biodiversity]). The limited results were then supplemented by searching within the reference lists of those initial studies, and also using the same search terms in Google Scholar. Our review was not limited to any specific regions, taxonomic groups, cultivation techniques, or research methods, but did only include studies on kelp aquaculture or those where ‘seaweed/macroalgae’ was used as a generic label. We also adopted the broader functional definition of ‘kelp’, which includes any large, brown, canopy-forming seaweed (i.e. Laminariales and Fucales sensu Steneck and Johnson 2014; Layton et al. 2020). Lastly, while we identified no research that focussed specifically on afforestation, we consider it here as having comparable effects as typical kelp farming, due to parallels in their operations and infrastructure.
The state of the literature
In total, we identified only 23 studies that examined some aspect of the biodiversity benefits of kelp or generic ‘seaweed’ aquaculture spanning from 2009 to 2021 (Table 1). Notably, the majority of these papers were reviews or industry reports (52%; n = 12), and there were relatively few experimental studies (48%, n = 11). Six (26%) of these papers reviewed literature on a global scale and ten (43%) dealt with ‘seaweed’ generally. Most of the reviews were also very general in scope, and often only broadly dealt with ecosystem services provided by different types of aquaculture (e.g. Theuerkauf et al. 2021). Thus, while biodiversity benefits were discussed widely, they were typically mentioned only as part of a suite of ecosystem services, rather than as the focus of the study. As such, ‘biodiversity benefits’ were often discussed peripherally and without detail. Notably, since we focussed only on English-language literature, we may have missed valuable data from relevant literature published in other languages (see Amano et al. 2021); especially that from east Asia where there is a long history and wealth of knowledge about seaweed aquaculture (Kim et al. 2017).
Attempted analyses across the identified experimental studies also suffered various limitations. Foremost, a lack of standardisation in research methods made comparisons challenging (something that has also been recognised by previous reviewers; Theuerkauf et al. 2021; Corrigan et al. 2022), as the studies looked at a variety of taxa, and used different experimental designs, control sites, and diversity metrics. For example, Radulovich et al. (2014) compared species richness and species-level abundances of fishes, macroinvertebrates, and algae at farms versus sandflat reef control sites, whereas Walls et al. (2016) compared species richness and total abundances of holdfast epifauna at farms relative to natural kelp forest control sites. Experiments were often conducted on relatively small temporal (e.g. weeks-months) and spatial (e.g. 1-3 × 60-280 m lines; ≤20 ha) scales, and on experimental farms that were not subject to disturbance from harvesting and commercial activity (e.g. Walls et al. 2016; Visch et al. 2020). These factors limit comparability across studies and to commercial kelp farming operations (Corrigan et al. 2022).
Kelp farm biodiversity and challenges
Physical and biological characteristics of kelp farm habitats
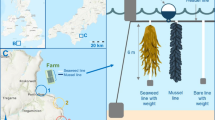
The majority of studies found that kelp farms can create habitat that may enhance local biodiversity, but with caveats (e.g. Wood et al. 2017; Hasselström et al. 2018; Visch et al. 2020; Theuerkauf et al. 2021). Like their natural counterparts, kelp farms drive a multitude of changes in their local physicochemical environment (Campbell et al. 2019), though there are important functional differences between kelp forests and farms. Natural kelp forests and their associated communities develop over years-decades among complex rocky substrates, whereas kelp farms are typically seeded and harvested over less-than annual periods and occur at the surface or shallow midwater with relatively little benthic structure/complexity (Fig. 1; Wood et al. 2017; Campbell et al. 2019; Walls et al. 2019). Nonetheless, the addition of the kelp and cultivation gear modifies local hydrodynamics and greatly increases the surface area available for colonisation by biota, while the growing kelp also change localised patterns of uptake, release, and cycling of carbon, nitrogen, and other nutrients (Campbell et al. 2019; Langton et al. 2019; Theuerkauf et al. 2021). The magnitude of these changes are largely unknown and are likely highly variable between farmed species and sites. For example, kelp farms typically result in reduced water movement within the farm but can also potentially increase external flows (Zeng et al. 2015; Zhang et al. 2020). Thus, by mediating local nutrients, light, and kinetic energy and, by providing structure and food subsidies, kelp farms can create shelter, spawning, and attachment sites, and also foraging opportunities for predators and prey (Campbell et al. 2019; Visch et al. 2020; Theuerkauf et al. 2021). Notably, these habitat benefits are enhanced as the cultivated kelp increase in size and complexity throughout the growing season, which can also coincide with seasonal increases in the activity of other marine organisms (Skjermo et al. 2014; Walls et al. 2017a). Experimental evidence has shown that this habitat can lead to increases in local biodiversity, with higher diversity of certain taxa at farms relative to control sites (e.g. Radulovich et al. 2014; Walls et al. 2016; Visch et al. 2020; Table 1).
Representative kelp forests (a, b) and kelp farms (c, d). Note that the kelp forests have dense and heterogenous biomass and are on complex rocky substrate, whereas the farms are midwater (as is typical), are comparatively homogenous, and have limited benthic substrate. Nonetheless, both habitats clearly provide surface area for colonisation by biota and create structure/biomass that might mediate local physicochemical conditions. The vertical yellow scale bar represents ~1 metre (photo credits a-d: S. Ling; Shutterstock; B. Skerry; C. Layton).
While kelp farms clearly do modify the environment to provide habitat for marine organisms, the effects of this on biodiversity are more nuanced and may not always equate to a net increase in biodiversity (e.g. due to different responses of mobile and sessile communities). Various factors influence the quality and type of habitat that kelp farms provide, and thereby their impacts on biodiversity. There are several main drivers that control the impact of aquaculture on ecosystems: the local environmental conditions, the intensity and scale of culture, the species cultivated, the cultivation gear used, and farm management practices (Hasselström et al. 2018; Theuerkauf et al. 2021; Corrigan et al. 2022). Differences between aquaculture operations in any of these drivers can lead to markedly different outcomes for biodiversity, which may not always be positive.
Many studies acknowledge that the biodiversity benefits of aquaculture vary in both time and space (e.g. Hasselström et al. 2018; Campbell et al. 2019; Theuerkauf et al. 2021) and can be minor or non-existent (e.g. Zhou 2012; Hehre and Meeuwig 2015; Walls et al. 2017b). A review by Theuerkauf et al. (2021) found that generic ‘seaweed’ farms were associated with large and highly variable increases in fish and mobile invertebrate richness, but with little increase in the abundance of these taxa. However, they found very few experimental studies specific to seaweed farms (n=8; mostly tropical seaweeds and relative to seagrass reference sites) and did not examine changes in species and community composition (see Table 1). Some of the variability in biodiversity responses reported previously may stem from there being few, non-standardised, experimental studies, but it is also likely inherent to kelp farm biodiversity. In other words, kelp farms are not guaranteed to provide enhanced biodiversity, but instead face a number of challenges that may limit their ability to provide the biodiversity benefits.
In the same way that kelp farms can modify their local environment to create habitat for some species, they can also drive major ecosystem changes and negative impacts on biodiversity (e.g. Wood et al. 2017; Campbell et al. 2019; Grebe et al. 2019). These impacts on biodiversity may arise through shading, physical obstruction, altered hydrodynamics, nutrient depletion, benthic enrichment, and even biochemical interactions (Eklöf et al. 2006; Walls et al. 2017b; Campbell et al. 2019). For instance, shading, nutrient depletion, and reduced water movement within kelp farms might all result in reductions to phytoplankton production and diversity due to competition with kelp for both light and nutrients (Campbell et al. 2019; Bach et al. 2021), as may complex biological interactions (e.g. Zhao et al. 2016).
In addition to ecosystem-level impacts, kelp farms can also have direct negative effects at the species level. For example, aquaculture infrastructure poses a threat to marine megafauna via entanglement (Benjamins et al. 2014; Grebe et al. 2019), as well as competition with megafauna and their exclusion from important habitat areas or migratory routes (Würsig et al. 2002; Markowitz et al. 2004). The response of megafauna to kelp farms will be highly variable across species and locations, but farming operations specifically present many of these same threats as other general forms of marine infrastructure if not managed appropriately (e.g. Benjamins et al. 2014; Langton et al. 2019).
The exact extent of these negative environmental impacts remains uncertain, but likely depends on the scale and intensity of farming combined with the carrying capacity of local ecosystems (Skjermo et al. 2014; Campbell et al. 2019; Grebe et al. 2019). Nonetheless, negative impacts on at least some taxa appear likely, meaning that any increases in biodiversity from kelp farms must also account for any associated losses and declines.
Novel communities of kelp farms
The complex interactions between positive and negative effects ultimately means that kelp farms often support novel communities from natural kelp forests (Campbell et al. 2019; Walls et al. 2019; Gentry et al. 2020). Rather, it seems they create ‘novel habitats’ that can facilitate certain species and inhibit others. This results in a species assemblage that is characteristic of the kelp farm rather than adjacent communities or natural analogues (Walls et al. 2016, 2019). These distinct communities arise from the unique physicochemical environment of kelp farms together with the biological environment that they create via ‘ecological priming’ and stochastic colonisation processes, where the initial seeding of farms with cultured kelp influences subsequent community assembly and succession (Walls et al. 2017a, 2019). Indeed, the species composition of macroinvertebrate communities in cultivated kelp holdfasts has been shown to be significantly different to those found in both adjacent natural kelp forests and on un-seeded ropes (Walls et al. 2016, 2019).
One consequence of kelp farms supporting novel communities is the risk that kelp farm habitat may facilitate invasive species and disease (Campbell et al. 2019; Grebe et al. 2019; Langton et al. 2019). Monocultures and artificial habitats are particularly susceptible to invasion and disease, especially as increased activity from commercial operations provides more transport vectors for marine pests (Stentiford et al. 2017; Campbell et al. 2019). As such, farms may act as ‘stepping-stones’ that facilitate the dispersal of invasive species or pathogens between farms and natural systems. Along with their ecological impacts, these organisms can also have severe commercial impacts and are widely recognised as a significant obstacle for the growth of the seaweed aquaculture industry (Cottier-Cook et al. 2016; Kim et al. 2017; Campbell et al. 2020). With such consequences, managers must consider the communities that will arise from the habitats they create.
Assessment of the species that make up kelp farm communities is also crucial to interpreting and evaluating the net biodiversity effects of farms, as broad diversity measures may be misleading. For instance, the addition of the cultivated kelp itself constitutes an increase in richness at the farm site, as does the establishment of any pests or fouling organisms (e.g. epiphytes and epifauna). To this end, increases in richness at kelp farm sites may not immediately reflect beneficial increases of diversity and functional groups. Indeed, much of the experimental research has examined kelp farm biodiversity in terms of fouling organisms (e.g. Walls et al. 2016, 2017a, 2019; Visch et al. 2020), but these species often degrade product quality and are commercially undesirable (Buschmann et al. 2017; Kim et al. 2017). Increased species biodiversity at kelp farms may therefore not always be positive for either commercial or restoration outcomes.
Kelp farm habitats also create and support distinct communities due to their inherent transient nature, that is, farmed biomass is expected to be harvested and removed. This further impacts any associated species and leads to the possibility of ‘ecological traps’ that might compound other negative impacts or negate prior positive effects. Ecological traps occur when organisms colonise habitats that may result in lowered fitness (Hale and Swearer 2016). Kelp harvest will obviously result in a radical loss of habitat for organisms that settle into and/or inhabit them (Wood et al. 2017; Grebe et al. 2019), meaning that kelp farms may act as ecological traps for species unable to disperse or survive cycles of commercial harvest and replanting (Skjermo et al. 2014; Theuerkauf et al. 2021; Corrigan et al. 2022).
As with much marine infrastructure, it remains unclear whether kelp farms increase species abundance and recruitment, or simply attract and amass organisms away from other habitats, like fish aggregating devices (Radulovich et al. 2014; Gentry et al. 2020; Corrigan et al. 2022). In particular, this could occur if kelp farms alter settlement dynamics by intercepting planktonic larvae and propagules that would otherwise disperse elsewhere (Barrett et al. 2022). This may drain surrounding populations and impact local/regional biodiversity, especially if they act as ecological traps.
Operational and management considerations
Given that kelp farms can have different biodiversity outcomes depending on a suite of operation-specific drivers, farms will likely require targeted management to maximise any biodiversity benefits (e.g. partial harvesting of biomass, fallowing; see Corrigan et al. 2022). However, it remains mostly unclear what those management needs are, and managing a farm to achieve positive biodiversity is likely to be especially costly and challenging in a commercial context where those ecosystem objectives must be integrated into sustainable business and harvest models (Buschmann et al. 2017; Campbell et al. 2019). This decision making may be further complicated by possible indirect effects of kelp aquaculture on local biodiversity, such as offsetting the wild harvest of kelp forests and therefore maintaining natural kelp forest communities. Similarly important considerations surround the provenance of local stock and whether the farmed species is naturally present in the surrounding environment, which is typically not the case for ocean afforestation. Even where enhanced biodiversity can be achieved, the kelp farm-associated organisms seem likely to be very different to those that occur in a natural kelp forest, meaning that environmental managers face difficult decisions about their priorities and values in terms of the taxa or ecosystems they choose to support. There is clearly a need to recognise the trade-offs and synergies between different ecosystem services and farming operations (Hasselström et al. 2018; Gentry et al. 2020; Barrett et al. 2022), and that kelp farms seem unlikely to be able to simultaneously provide all of the services for which they have been purported.
Whether these challenges will dissuade commercial aquaculture managers from seeking biodiversity benefits remains unclear, and regulatory incentives might play an important role in setting these priorities (Theuerkauf et al. 2021; Corrigan et al. 2022). Regardless, commercial kelp aquaculture has specific goals that may not align with direct ecological or restoration outcomes and requires the periodic destruction of any habitat that is created. Therefore, all stakeholders must decide and clarify what they want kelp farms to achieve and produce in order to avoid overpromising and ensure that commercial and conservation objectives are not undermined.
Conclusions and the future
Knowledge gaps and research priorities
A final and fundamental challenge for kelp farm biodiversity is that the industry and the world’s oceans are changing rapidly, and farms may have a very different ability to provide habitat as production practices develop. In particular, disease and climate change pose significant obstacles for the future of kelp farming, whilst cultivar development and emerging technologies like genetic manipulation are set to help respond to these threats and optimise commercial yields (Cottier-Cook et al. 2016; Kim et al. 2017). Different strains and hybrid kelps are already being developed (e.g. Li et al. 2016), and such cultivars may create distinct habitats through differences in their life history, growth form, and environmental tolerances. Similarly, the movement of kelp farms offshore has been suggested to help minimise social and environmental concerns (Kim et al. 2017; Bak et al. 2020), and afforestation efforts are typically focused offshore (Bach et al. 2021; Boyd et al. 2022). The effect of offshore kelp farms and afforestation on biodiversity is understudied, though the lack of recruitment sources and the unique physical and ecological conditions in this exposed environment suggest very different outcomes for biodiversity (e.g. Bach et al. 2021; Boyd et al. 2022).
Further research is essential to address the gaps in the literature and enable informed decisions about when and where the biodiversity services of kelp farms could be useful (Wood et al. 2017; Grebe et al. 2019; Theuerkauf et al. 2021). Foremost among these research priorities is a need for more experimental work, as the disproportionate number of broad reviews and technical reports means that assessments of kelp farm biodiversity are at risk of being oversimplified, repetitive, and based on only a few key sources. Coupled with this experimental work is the need for standardised methods of quantifying biodiversity on kelp farms relative to appropriate reference systems (Corrigan et al. 2022). A standardised monitoring approach will enable greater comparability across studies, which is essential to understand the effects of kelp farms in different settings. At present, the lack of standardised experimental research on this topic (see Table 1) limits the potential to conduct a rigorous quantitative analysis of the literature. We especially emphasise the importance of expanding surveying efforts over greater spatial and temporal scales, for example, long-term monitoring to account for the impacts of periodic harvesting on farm biodiversity (Walls et al. 2017b; Wood et al. 2017). Monitoring should also incorporate diversity transparently across various taxonomic and functional groups, in order to develop a holistic picture of community changes and better understand the effects of kelp farms on taxa that have been poorly represented in past research (e.g. microorganisms, plankton, and megafauna).
Conclusion
While kelp farms do create novel habitat for some associated organisms, the potential for farms to improve biodiversity and achieve restoration or environmental outcomes is variable and uncertain, and perhaps at odds with commercial objectives that necessitate harvest. It appears likely that the biodiversity benefits of kelp farms need to be evaluated on an operation-specific basis, and that they will require targeted management to be achieved. Ultimately, even if kelp farms do enhance biodiversity and support diverse communities of organisms, this may not benefit either the farms or their associated organisms, in the case of marine pests or ecological traps. Farms may not be forests, so we should be cautious about treating them as equivalent. Rather than dealing with trade-offs and compromised outcomes, it ultimately may be best to recognise farms for their own distinct and valuable services and focus farming operations on the sustainable production of biomass, whilst supporting restoration and conservation practices to pursue biodiversity and environmental goals.
References
Amano T, Berdejo-Espinola V, Christie AP et al (2021) Tapping into non-English-language science for the conservation of global biodiversity. PLoS Biol 19:e3001296
Bach LT, Tamsitt V, Gower J, Hurd CL, Raven JA, Boyd PW (2021) Testing the climate intervention potential of ocean afforestation using the Great Atlantic Sargassum Belt. Nat Commun 12:2556
Bak UG, Gregersen Ó, Infante J (2020) Technical challenges for offshore cultivation of kelp species: lessons learned and future directions. Bot Mar 63:341–353
Barrett LT, Theuerkauf SJ, Rose JM, Alleway HK, Bricker SB, Parker M, Petrolia DR, Jones RC (2022) Sustainable growth of non-fed aquaculture can generate valuable ecosystem benefits. Ecosyst Serv 53:101396
Bax N, Novaglio C, Maxwell KH, Meyers K, McCann J, Jennings S, Frusher S, Fulton EA, Nursey-Bray M, Fischer M, Anderson K, Layton C, Emad GR, Alexander KA, Rousseau Y, Lunn Z, Carter CG (2022) Ocean resource use: building the coastal blue economy. Rev Fish Biol Fish 32:189–207
Benjamins S, Harnois V, Smith H, Johanning L, Greenhill L, Carter C, Wilson B (2014) Understanding the potential for marine megafauna entanglement risk from marine renewable energy developments. Scottish Natural Heritage Commissioned Report No. 791, 95 p
Bennett S, Wernberg T, Connell SD, Hobday AJ, Johnson CR, Poloczanska ES (2015) The ‘Great Southern Reef’: social, ecological and economic value of Australia’s neglected kelp forests. Mar Freshwat Res 67:47–56
Boyd PW, Bach LT, Hurd CL, Paine E, Raven JA, Tamsitt V (2022) Potential negative effects of ocean afforestation on offshore ecosystems. Nat Ecol Evol 6:675–683
Buschmann AH, Camus C, Infante J, Neori A, Israel Á, Hernández-González MC, Pereda SV, Gomez-Pinchetti JL, Golberg A, Tadmor-Shalev N, Critchley AT (2017) Seaweed production: overview of the global state of exploitation, farming and emerging research activity. Eur J Phycol 52:391–406
Campbell I, Macleod A, Sahlmann C, Neves L, Funderud J, Øverland M, Hughes AD, Stanley M (2019) The environmental risks associated with the development of seaweed farming in Europe - prioritizing key knowledge gaps. Front Mar Sci 6:107
Campbell I, Kambey CSB, Mateo JP, Rusekwa SB, Hurtado AQ, Msuya FE, Stentiford GD, Cottier-Cook EJ (2020) Biosecurity policy and legislation for the global seaweed aquaculture industry. J Appl Phycol 32:2133–2146
Corrigan S, Brown AR, Ashton IGC, Smale DA, Tyler CR (2022) Quantifying habitat provisioning at macroalgal cultivation sites. Rev Aquacult 14:1671–1694
Costa-Pierce B, Chopin T (2021) The hype, fantasies and realities of aquaculture development globally and in its new geographies. World Aquac 52:23–35
Cottier-Cook E-J, Nagabhatla N, Badis Y, et al (2016) Safeguarding the future of the global seaweed aquaculture industry. United Nations University (INWEH) and Scottish Association for Marine Science Policy Brief. ISBN 978-92-808-6080-1. 12pp
Eger A, Marzinelli E, Baes R et al (2021) The economic value of fisheries, blue carbon, and nutrient cycling in global marine forests. EcoEvoRxiv. https://doi.org/10.32942/osf.io/n7kjs
Eklöf J, Kautsky N, Henriksson R (2006) Effects of tropical open-water seaweed farming on seagrass ecosystem structure and function. Mar Ecol Prog Ser 325:73–84
FAO (2018) The State of World Fisheries and Aquaculture 2018: Meeting the sustainable development goals. FAO, Rome
FAO (2020) The State of World Fisheries and Aquaculture 2020: Sustainability in action. FAO, Rome
Filbee-Dexter K, Wernberg T, Barreiro R, Coleman MA, de Bettignies T, Feehan CJ, Franco JN, Hasler B, Louro I, Norderhaug KM, Staehr PAU, Tuya F, Verbeek J (2022) Leveraging the blue economy to transform marine forest restoration. J Phycol 58:198–207
Gallagher JB, Shelamoff V, Layton C (2022) Seaweed ecosystems may not mitigate CO2 emissions. ICES J Mar Sci 79:585–592
Gao Q, Ling J, Tang B, Sun P, Jiang Y (2021) Effects of facilities associated with marine ranching on zooplankton community: a case study in Xiangshan Bay, China. J Fish Sci China 28:411–419
Gentry RR, Alleway HK, Bishop MJ, Gillies CL, Waters T, Jones R (2020) Exploring the potential for marine aquaculture to contribute to ecosystem services. Rev Aquacult 12:499–512
Grebe GS, Byron CJ, St Gelais A, Kotowicz DM, Olson TK (2019) An ecosystem approach to kelp aquaculture in the Americas and Europe. Aquacult Rep 15:100215
Hale R, Swearer S (2016) Ecological traps: current evidence and future directions. Proc R Soc B 283:20152647
Hasselström L, Visch W, Gröndahl F, Nylund GM, Pavia H (2018) The impact of seaweed cultivation on ecosystem services - a case study from the west coast of Sweden. Mar Pollut Bull 133:53–64
Hehre EJ, Meeuwig JJ (2015) Differential response of fish assemblages to coral reef-based seaweed farming. PLoS One 10:e0118838
Kelly J (2020) The Official Blueprint for Seaweed in Australia. AgriFutures Australia, Canberra
Kim JK, Yarish C, Hwang EK, Park M, Kim Y (2017) Seaweed aquaculture: cultivation technologies, challenges and its ecosystem services. Algae 32:1–13
Krumhansl KA, Okamoto DK, Rassweiler A et al (2016) Global patterns of kelp forest change over the past half-century. Proc Natl Acad Sci U S A 113:13785–13790
Langton R, Augyte S, Price N, Forster J, Noji T, Grebe G, St Gelais A, Byron CJ (2019) An ecosystem approach to the culture of seaweed. NOAA Tech. Memo. NMFS-F/SPO-195, 24 p
Layton C, Coleman MA, Marzinelli EM, Steinberg PD, Swearer SE, Vergés A, Wernberg T, Johnson CR (2020) Kelp forest restoration in Australia. Front Mar Sci 7:74
Li X, Zhang Z, Qu S, Liang G, Sun J, Zhao N, Cui C, Cao Z, Li Y, Pan J, Yu S, Wang Q, Li X, Luo S, Song S, Guo L, Yang G (2016) Improving seedless kelp (Saccharina japonica) during its domestication by hybridizing gametophytes and seedling-raising from sporophytes. Sci Rep 6:21255
Markowitz T, Harlin A, Würsig B, McFadden C (2004) Dusky dolphin foraging habitat: Overlap with aquaculture in New Zealand. Aquat Conserv 14:133–149
Peteiro C, Freire Ó (2013) Epiphytism on blades of the edible kelps Undaria pinnatifida and Saccharina latissima farmed under different abiotic conditions. J World Aquac Soc 44:706–715
Radulovich R, Umanzor S, Cabrera R, Mata R (2014) Tropical seaweeds for human food, their cultivation and its effect on biodiversity enrichment. Aquaculture 436:40–46
Skjermo J, Aasen I, Arff J, Broch OJ, Carvajal AK et al (2014) A new Norwegian bioeconomy based on cultivation and processing of seaweeds: Opportunities and R&D needs. SINTEF Fisheries and Aquaculture, Trondheim
Smale DA, Burrows MT, Moore P, O’Connor N, Hawkins SJ (2013) Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol Evol 3:4016–4038
Steneck RS, Johnson CR (2014) Kelp forests: dynamic patterns, processes, and feedbacks. In: Bertness MD, Bruno JF, Silliman BR, Stachowicz JJ (eds) Marine community ecology and conservation. Sinauer Associates, Inc., Massachusetts, pp 315–336
Stentiford GD, Sritunyalucksana K, Flegel TW, Williams BA, Withyachumnarnkul B, Itsathitphaisarn O, Bass D (2017) New paradigms to help solve the global aquaculture disease crisis. PLOS Pathog 13:e1006160
Theuerkauf SJ, Barrett LT, Alleway HK, Costa-Pierce BA, St. Gelais A, Jones RC (2021) Habitat value of bivalve shellfish and seaweed aquaculture for fish and invertebrates: Pathways, synthesis and next steps. Rev Aquacult 14:54–72
Visch W, Kononets M, Hall POJ, Nylund GM, Pavia H (2020) Environmental impact of kelp (Saccharina latissima) aquaculture. Mar Pollut Bull 155:110962
Walls AM, Kennedy R, Fitzgerald RD, Blight AJ, Johnson MP, Edwards MD (2016) Potential novel habitat created by holdfasts from cultivated Laminaria digitata: assessing the macroinvertebrate assemblages. Aquacult Env Interact 8:157–169
Walls AM, Edwards MD, Firth LB, Johnson MP (2017a) Successional changes of epibiont fouling communities of the cultivated kelp Alaria esculenta: predictability and influences. Aquacult Env Interact 9:57–71
Walls AM, Kennedy R, Edwards MD, Johnson MP (2017b) Impact of kelp cultivation on the ecological status of benthic habitats and Zostera marina seagrass biomass. Mar Pollut Bull 123:19–27
Walls AM, Edwards MD, Firth LB, Johnson MP (2019) Ecological priming of artificial aquaculture structures: kelp farms as an example. J Mar Biol Assoc UK 99:729–740
Wood D, Capuzzo E, Kirby D, Mooney-McAuley K, Kerrison P (2017) UK macroalgae aquaculture: What are the key environmental and licensing considerations? Mar Policy 83:29–39
Wright LS, Pessarrodona A, Foggo A (2022) Climate-driven shifts in kelp forest composition reduce carbon sequestration potential. Glob Chang Biol 28:5514–5531
Würsig B, Gailey G, Stickney R, Mcvey J (2002) Marine mammals and aquaculture: conflicts and potential resolutions. In: Stickney RR, McVey JP (eds) Responsible marine aquaculture. CAB International, Wallingford, pp 45–59
Zeng D, Huang D, Qiao X, He Y, Zhang T (2015) Effect of suspended kelp culture on water exchange as estimated by in situ current measurement in Sanggou Bay, China. J Mar Syst 149:14–24
Zhang J, Hansen PK, Fang J, Wang W, Jiang Z (2009) Assessment of the local environmental impact of intensive marine shellfish and seaweed farming—application of the MOM system in the Sungo Bay, China. Aquaculture 287:304–310
Zhang Z, Huang H, Liu Y, Bi H, Yan L (2020) Numerical study of hydrodynamic conditions and sedimentary environments of the suspended kelp aquaculture area in Heini Bay. Estuar Coast Shelf Sci 232:106492
Zhao L, Zhao Y, Xu J, Zhang W, Huang L, Jiang Z, Fang J, Xiao T (2016) Distribution and seasonal variation of picoplankton in Sanggou Bay, China. Aquacult Env Interact 8:261–271
Zhou J (2012) Impacts of mariculture practices on the temporal distribution of macrobenthos in Sandu Bay, South China. Chin J Ocean Limnol 30:388–396
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Cayne Layton receives funding support from The Nature Conservancy California Oceans Program.
Author information
Authors and Affiliations
Contributions
Conceptualisation: Hunter Forbes, Cayne Layton; Methodology: Hunter Forbes, Cayne Layton; Investigation: Hunter Forbes, Victor Shelamoff, Wouter Visch, Cayne Layton; Writing - original draft preparation: Hunter Forbes; Writing – review and editing: Hunter Forbes, Victor Shelamoff, Wouter Visch, Cayne Layton; Supervision: Cayne Layton
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Forbes, H., Shelamoff, V., Visch, W. et al. Farms and forests: evaluating the biodiversity benefits of kelp aquaculture. J Appl Phycol 34, 3059–3067 (2022). https://doi.org/10.1007/s10811-022-02822-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02822-y