Abstract
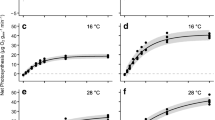
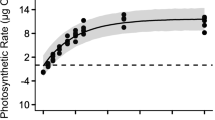
The combined effects of temperature and irradiance and the influences of desiccation and salinity on the photochemical efficiency in a subtropical red alga, Phycocalidia tanegashimensis (= Pyropia tanegashimensis, Bangiaceae) from Tanegashima Island, Japan, were determined to reveal how this species has adapted to its habitat in the splash zone. Continuous 6-h exposure to irradiance of 200 (low) and 1000 (high) µmol photons m−2 s−1 at 12, 20, and 28 °C showed a decline in the effective quantum yields (ΔF/Fm′) of photosystem II (PSII) during the exposures; nevertheless, the maximum quantum yields (Fv/Fm) of PSII measured in subsequent 14-h dim-light acclimation almost returned to initial values at 20 and 28 °C, revealing its high capacity to recovery. In contrast, those under both low and high irradiances at 12 °C did not recover to initial values even after 14-h dim-light acclimation, signifying enhanced inhibition under irradiance at low temperature. The response to continuous desiccation (~ 480 min) under 50% humidity at 24 °C showed that the ΔF/Fm′ decreased with decreasing absolute water content (AWC). However, for the samples with an AWC above 10%, ΔF/Fm′ mostly recovered to initial levels after subsequent 1-day rehydration in seawater, suggesting relatively strong tolerance to desiccation. This alga also tolerated a broad range of salinity (i.e., 10–60 psu) under 3-day exposures. The adaptations of P. tanegashimensis to relatively high irradiance, warm temperature, and a strong osmotic (desiccation and salinity) tolerance may explain its high capacity to flourish in the splash zone in the subtropical environment of Japan.




Similar content being viewed by others
Data availability
The datasets generated in the present study are available from the corresponding author on reasonable request.
References
Abbott IA (2004) Marine red algae of the Hawaiian Islands. Bishop Museum Press, Honolulu
Abe M, Kobayashi M, Fujiyoshi E, Tamaki M, Kikuchi N, Murase N (2013) Use of PCR-RFLP for the discrimination of Japanese Porphyra and Pyropia species (Bangiales, Rhodophyta). J Appl Phycol 25:225–232
Allakhverdiev SI, Murata N (2004) Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage-repair cycle of photosystem II in Synechocystis sp. PCC 6803. Biochim Biophys Acta 1657:23–32
Beer S, Biörk M, Beardall J (2014) Photosynthesis in the marine environment. Wiley-Blackwell, Ames
Bessho K, Iwasa Y (2009) Heteromorphic and isomorphic alternations of generations in macroalgae as adaptations to a seasonal environment. Evol Ecol Res 11:691–711
Bessho K, Iwasa Y (2010) Optimal seasonal schedules and the relative dominance of heteromorphic and isomorphic life cycles in macroalgae. J Theor Biol 267:201–212
Bessho K, Iwasa Y (2012) Variability in the evolutionarily stable seasonal timing of germination and maturation of annuals and the mode of competition. J Theor Biol 304:66–80
Borlongan IA, Nishihara GN, Shimada S, Terada R (2017) Effects of temperature and PAR on the photosynthesis of Kappaphycus sp. (Solieriaceae, Rhodophyta) from Okinawa, Japan, as the northern limit of native Kappaphycus distribution in the western Pacific. Phycologia 56:444–453
Contreras-Porcia L, Thomas D, Flores V, Correa JA (2011) Tolerance to oxidative stress induced by desiccation in Porphyra columbina (Bangiales, Rhodophyta). J Exp Bot 62:1815–1829
Davison IR, Peason GA (1996) Stress tolerance in intertidal seaweeds. J Phycol 32:197–211
Drew KM (1949) Conchocelis-phase in the life-history of Porphyra umbilicalis (L.) Kütz. Nature 164:748–749
Drew KM (1954) Life-history of Porphyra. Nature 173:1243–1244
Dring MJ, Brown FA (1982) Photosynthesis of intertidal brown algae during and after periods of emersion: a renewed search for physiological causes of zonation. Mar Ecol Prog Ser 8:301–308
Dumilag RV, Monotilla WD (2018) Molecular diversity and biogeography of Philippine foliose Bangiales (Rhodophyta). J Appl Phycol 30:173–186
Dumilag RV, Aguinaldo Z-ZA, Mintu CB, Quinton MP, Ame EC, Andres RC, Monotilla WD, Yap SL, Cao EP, Vital PG, Fontanilla IKC (2017) A review of the current taxonomic status of foliose Bangiales (Rhodophyta) in the Philippines. Phytotaxa 312:47–59
Eggert A (2012) Seaweed responses to temperature. In: Wiencke C, Bischof K (eds) Seaweed biology. Novel insights into ecophysiology, ecology and utilization. Springer, Berlin, pp 48–66
Fukumoto R, Nishihara GN, Endo H, Terada R (2018) The photosynthetic responses to PAR and temperature including chilling-light stress on the heteromorphic life history stages of a brown alga, Cladosiphon okamuranus (Chordariaceae) from Ryukyu Islands, Japan. Phycol Res 66:209–217
Fukumoto R, Nishihara GN, Endo H, Terada R (2019) Effect of photosynthetically active radiation and temperature on the photosynthesis of two heteromorphic life history stages of a temperate edible brown alga, Cladosiphon umezakii (Chordariaceae, Ectocarpales), from Japan. J Appl Phycol 31:1259–1270
Gao S, Wang G (2012) The enhancement of cyclic electron flow around photosystem I improves the recovery of severely desiccated Porphyra yezoensis (Bangiales, Rhodophyta). J Exp Bot 63:4349–4358
Gao S, Shen S, Wang G, Niu J, Lin A, Pan G (2011) PSI-driven cyclic electron flow allows intertidal macro-algae Ulva sp. (Chlorophyta) to survive in desiccated conditions. Plant Cell Physiol 52:885–893
Gao S, Niu J, Chen W, Wang G, Xie X, Pan G, Gu W, Zhu D (2013) The physiological links of the increased photosystem II activity in moderately desiccated Porphyra haitanensis (Bangiales, Rhodophyta) to the cyclic electron flow during desiccation and re-hydration. Photosyn Res 116:45–54
Holzinger A, Karsten U (2013) Desiccation stress and tolerance in green algae: consequences for ultrastructure, physiological, and molecular mechanisms. Front Plant Sci 4:327
Hurd CL, Harrison PJ, Bischof K, Lobban CS (2014) Seaweed ecology and physiology, 2nd edn. Cambridge University Press, Cambridge
Ito T, Borlongan IA, Nishihara GN, Endo H, Terada R (2021) The effects of irradiance, temperature, and desiccation on the photosynthesis of a brown alga, Sargassum muticum (Fucales), from a native distributional range in Japan. J Appl Phycol 33. https://doi.org/10.1007/s10811-021-02425-z
Ji Y, Tanaka J (2002) Effect of desiccation on the photosynthesis of seaweeds from the intertidal zone in Honshu, Japan. Phycol Res 50:145–153
Karsten U (2012) Seaweed acclimation to salinity and desiccation stress. In: Wiencke C, Bischof K (eds) Seaweed biology. Novel insights into ecophysiology, ecology and utilization. Springer, Berlin, pp 87–107
Kim KY, Garbary DJ (2007) Photosynthesis in Codium fragile (Chlorophyta) from a Nova Scotia estuary: responses to desiccation and hyposalinity. Mar Biol 151:99–107
Kirst GO (1990) Salinity tolerance of eukaryotic marine algae. Annu Rev Plant Physiol Plant Mol Biol 41:21–53
Kokubu S, Nishihara GN, Watanabe Y, Tsuchiya Y, Amano Y, Terada R (2015) The effect of irradiance and temperature on the photosynthesis of a native brown alga, Sargassum fusiforme (Fucales) from Kagoshima, Japan. Phycologia 54:235–247
Nelson WA, Brodie J, Guiry MD (1999) Terminology used to describe reproduction and life history stages in the genus Porphyra (Bangiales, Rhodophyta). J Appl Phycol 11:407–410
Santiañez WJE, Wynne MJ (2020) Proposal of Phycocalidia Santiañez & M.J.Wynne nom. nov. to replace Calidia L.-E.Yang & J. Brodie nom. illeg. (Bangiales, Rhodophyta). Notulae Algarum 140:1–3
Shimabukuro H, Terada R, Sotobayashi J, Nishihara GN, Noro T (2007) Phenology of Sargassum duplicatum (Fucales, Phaeophyceae) from the southern coast of Satsuma Peninsula, Kagoshima, Japan. Nippon Suisan Gakkaishi 73:454–460 (in Japanese with English abstract)
Shinmura I (1974) Porphyra tanegashimensis, a new species of Rhodophyceae from Tanegashima Island in southern Japan. Bull Jpn Soc Sci Fish 40:735–749
Sutherland JE, Lindstrom SC, Nelson WA, Brodie J, Lynch MD, Hwang MS, Choi HG, Miyata M, Kikuchi N, Cliveira MC, Farr T, Neefus C, Mols-Mortensen MD, Müller K (2011) A new look at an ancient order: generic revision of the Bangiales (Rhodophyta). J Phycol 47:1131–1151
Tanaka T, Hô PH (1962) Notes of some marine algae from Viet-Nam - I. Mem Fac Fish Kagoshima Univ 11:24–40
Terada R, Watanabe Y (2017) Seaweeds and coastal environment in the Osumi Islands. In: Kawai K, Terada R, Kuwahara S (eds) The Osumi Islands: Culture, society, industry and nature. Kagoshima University Research Center for the Pacific Islands (KURCPI). Hokuto Shobo Publishing, Tokyo, pp 104–108
Terada R, Borlongan IA, Watanabe Y, Nishihara GN, Endo H, Shimada S (2018a) The combined effects of PAR and temperature including the chilling-light stress on the photosynthesis of a temperate brown alga, Sargassum patens (Fucales), based on field and laboratory measurements. J Appl Phycol 30:1893–1904
Terada R, Nakazaki Y, Borlongan IA, Endo H, Nishihara GN (2018b) Desiccation effect on the PSII photochemical efficiency of cultivated Japanese Caulerpa lentillifera under the shipping package environment. J Appl Phycol 30:2533–2588
Terada R, Nakahara K, Borlongan IA, Watanabe Y, Mine T, Morikawa T, Igari T, Nishi H, Endo H, Nishihara GN (2019) Combined effects of irradiance and temperature on the PSII photochemical efficiency in the heteromorphic life history stages of cultivated Pyropia (Bangiales): P. yezoensis f. narawaensis and P. tenera from Japan. J Appl Phycol 31:1251–1257
Terada R, Nakashima Y, Borlongan IA, Shimabukuro H, Kozono J, Endo H, Shimada S, Nishihara GN (2020a) Photosynthetic activity including the thermal- and chilling-light sensitivities of a temperate Japanese brown alga Sargassum macrocarpum. Phycol Res 68:70–79
Terada R, Yuge T, Watanabe Y, Mine T, Morikawa T, Nishihara GN (2020b) Chronic effects of three different stressors, irradiance, temperature, and desiccation on the PSII photochemical efficiency in the heteromorphic life-history stages of cultivated Pyropia yezoensis f. narawaensis (Bangiales) from Japan. J Appl Phycol 32:3273–3284
Terada R, Nishihara GN, Arimura K, Watanabe Y, Mine T, Morikawa T (2021a) Photosynthetic response of a cultivated red alga, Neopyropia yezoensis f. narawaensis (= Pyropia yezoensis f. narawaensis; Bangiales, Rhodophyta) to dehydration stress differs with between two heteromorphic life-history stages. Algal Res 55: 102262
Terada R, Takaesu M, Borlongan IA, Nishihara GN (2021b) The photosynthetic performance of a cultivated Japanese green alga Caulerpa lentillifera in response to three different stressors, temperature, irradiance, and desiccation. J Appl Phycol 33. https://doi.org/10.1007/s10811-021-02439-7 (online)
Wang WJ, Wang FJ, Zhu JY, Sun XT, Yao CY, Xu P (2011) Freezing tolerance of Porphyra yezoensis (Bangiales, Rhodophyta) gametophyte assessed by chlorophyll fluorescence. J Appl Phycol 23:1017–1022
Watanabe Y, Nishihara GN, Tokunaga S, Terada R (2014) Effect of irradiance and temperature on the photosynthesis of a cultivated red alga, Pyropia tenera (= Porphyra tenera), at the southern limit of distribution in Japan. Phycol Res 62:187–196
Watanabe Y, Yamada H, Mine Y, Kawamura Y, Nishihara GN, Terada R (2016) The response of photosynthesis of Pyropia yezoensis f. narawaensis to a thermal and PAR gradient varies with the life-history stage. Phycologia 55:665–672
Watanabe Y, Morikawa T, Mine T, Kawamura Y, Nishihara GN, Terada R (2017) Chronological change and the potential of recovery on the photosynthetic efficiency of Pyropia yezoensis f. narawaensis (Bangiales) during the sporelings frozen storage treatment in the Japanese Nori cultivation. Phycol Res 65:265–271
Wiltens J, Schreiber U, Vidaver W (1978) Chlorophyll fluorescence induction: an indicator of photosynthetic activity in marine algae understanding desiccation. Can J Bot 56:2787–2794
Xu G, Terada R, Watanabe Y, Nishihara GN (2021) Temperature characteristics on the growth and photosynthesis of a red alga, Phycocalidia tanegashimensis (= Pyropia tanegashimensis, Bangiales) reveal adaptation to subtropical environments due to year-round occurrence of the macroscopic gametophyte. J Appl Phycol 33. https://doi.org/10.1007/s10811-021-02426-y (online)
Yang LE, Deng YY, Xu GP, Russell S, Lu QQ, Brodie J (2020) Redefining Pyropia (Bangiales, Rhodophyta): four new genera, resurrection of Porphyrella and description of Calidia pseudolobata sp. nov. from China. J Phycol 56:862–879
Acknowledgements
We wish to express our thanks to Ms. Moe Takaesu, Faculty of Fisheries, Kagoshima University, for her kind assistance in the experiments. RT also expresses his gratitude to Dr. Iwao Shinmura for his great pioneering study in P. tanegashimensis and wishes continued good health and long life. The first author GX conducted this research as an international technical trainee at the United Graduate School of Agricultural Sciences, Kagoshima University.
Funding
This research was supported in part by the Overseas Technical Trainee Exchange Program of the Kagoshima Prefecture Government and by the Grant-in-Aid for Scientific Research (B; # 20H03076) from Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All authors have provided consent.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10811_2021_2498_MOESM1_ESM.jpg
Supplementary Fig. 1 The habitat of the red alga, Phycocalidia tanegashimensis (= Pyropia tanegashimensis) from Tanegashima Island, Japan. A photo was taken during the high tide with heavy wave motion.ementary file1 (JPG 1009 KB)
Rights and permissions
About this article
Cite this article
Xu, G., Terada, R., Watanabe, Y. et al. The occurrence of Phycocalidia tanegashimensis (Bangiaceae) in the splash zone may be related to the tolerance of photochemical efficiency to temperature, irradiance, desiccation, and salinity. J Appl Phycol 33, 3427–3435 (2021). https://doi.org/10.1007/s10811-021-02498-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02498-w