Abstract
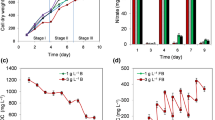
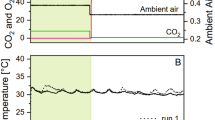
Solar cultivation of microalgae in photobioreactors is a valuable bioprocess for the sustainable production of commercially useful metabolites. However, the conventional culture temperature control method in solar closed photobioreactors of evaporative cooling is neither economical nor sustainable. In this study, a novel spectrally selective, insulated glazed flat plate (IGP) photobioreactor employing an infrared reflecting system embedded in the illumination surface was used for cultivation of Nannochloropsis sp. The impact of the temperature control technology on protein, lipid, carbohydrate content and fatty acid profile of Nannochloropsis sp. was investigated and compared to closed photobioreactors using passive evaporative cooling (PEC) and an infrared reflecting film (IRF) on the surface as well as an open raceway pond (ORP). Among all cultivation systems tested, the biochemical composition of biomass (mg g−1 organic biomass) showed a general trend of lipid > protein > carbohydrate, with no large variation of each across treatments. However, the areal and volumetric productivities of these constituents were significantly higher in the photobioreactors than in the ORP; results consistent with biomass productivity data. Of the major saturated and monounsaturated fatty acids present, only the proportion of C16:0, which is 24% higher in the photobioreactors than in the ORP, changed significantly among cultivation systems. The highest content of high-value dietary fatty acids, eicosapentaenoic acid (EPA, C20:5n-3; 15.5%) and ϒ-linolenic acid (C18:3n-6; 8%) were found in the ORP but were similar to that produced in the IGP (15.9 and 3.4%, respectively). Among all photobioreactors, the IGP had the least diel temperature changes and an EPA content that was 21% higher than PEC. Photobioreactors constructed with spectrally selective materials effectively allow management of internal reactor temperature with no significant negative impact on biochemical and fatty acid profiles of microalgae.



Similar content being viewed by others
References
Barsanti L, Gualtieri P (2018) Is exploitation of microalgae economically and energetically sustainable? Algal Res 31:107–115
Bechet Q, Shilton A, Fringer OB, Munoz R, Guieysse B (2010) Mechanistic modeling of broth temperature in outdoor photobioreactors. Environ Sci Technol 44:2197–2203
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. Aust J Biotechnol 70:313–321
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Borowitzka MA (2016) Algal physiology and large-scale outdoor cultures of microalgae. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 601–652
Boruff BJ, Moheimani NR, Borowitzka MA (2015) Identifying locations for large-scale microalgae cultivation in Western Australia: a GIS approach. Appl Energy 149:379–391
Cai T, Park SY, Racharaks R, Li Y (2013) Cultivation of Nannochloropsis salina using anaerobic digestion effluent as a nutrient source for biofuel production. Appl Energy 108:486–492
Estime B, Ren D, Sureshkumar R (2015) Effects of plasmonic film filters on microalgal growth and biomass composition. Algal Res 11:85–89
Global Organisation for EPA and DHA Technical Guidance Document (2019) https://www.goedomega3.com/goed-monograph. Accessed 22 September 2019
Goetz V, Le Borgne F, Pruvost J, Plantard G, Legrand J (2011) A generic temperature model for solar photobioreactors. Chem Eng J 175:443–449
Guillard RR, Ryther JH (1962) Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186
He Q, Yang H, Wu L, Hu C (2015) Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour Technol 191:219–228
Hindersin S, Leupold M, Kerner M, Hanelt D (2013) Irradiance optimization of outdoor microalgal cultures using solar tracked photobioreactors. Bioprocess Biosyst Eng 36:345–355
Lang I, Hodac L, Friedl T, Feussner I (2011) Fatty acid profiles and their distribution patterns in microalgae: a comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol 11:124
Li X, Liu J, Chen G, Zhang J, Wang C, Liu B (2019) Extraction and purification of eicosapentaenoic acid and docosahexaenoic acid from microalgae: a critical review. Algal Res 43:101619
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Ma Y, Wang Z, Yu C, Yin Y, Zhou G (2014) Evaluation of the potential of 9 Nannochloropsis strains for biodiesel production. Bioresour Technol 167:503–509
Moheimani NR, Borowitzka MA, Isdepsky A, Fon Sing F (2013) Standard methods for measuring growth of algae and their composition. In: Algae for biofuels and energy. Springer, Dordrecht pp. 265–284
Müller P, Li X-P, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Nwoba EG, Parlevliet DA, Laird DW, Alameh K, Moheimani NR (2019a) Light management technologies for increasing algal photobioreactor efficiency. Algal Res 39:101433
Nwoba EG, Parlevliet DA, Laird DW, Alameh K, Moheimani NR (2020a) Outdoor phycocyanin production in a standalone thermally-insulated photobioreactor. Bioresour Technol:123865
Nwoba EG, Parlevliet DA, Laird DW, Alameh K, Moheimani NR (2020b) Pilot-scale self-cooling microalgal closed photobioreactor for biomass production and electricity generation. Algal Res 45:101731
Nwoba EG, Parlevliet DA, Laird DW, Vadiveloo A, Alameh K, Moheimani NR (2019b) Can solar control infrared blocking films be used to replace evaporative cooling for growth of Nannochloropsis sp. in plate photobioreactors? Algal Res 39:101441
Pruvost J, Le Gouic B, Lepine O, Legrand J, Le Borgne F (2016) Microalgae culture in building-integrated photobioreactors: biomass production modelling and energetic analysis. Chem Eng J 284:850–861
Raes E, Isdepsky A, Muylaert K, Borowitzka M, Moheimani N (2014) Comparison of growth of Tetraselmis in a tubular photobioreactor (Biocoil) and a raceway pond. J Appl Phycol 26:247–255
Shene C, Chisti Y, Vergara D, Burgos-Díaz C, Rubilar M, Bustamante M (2016) Production of eicosapentaenoic acid by Nannochloropsis oculata: effects of carbon dioxide and glycerol. J Biotechnol 239:47–56
Suzuki H, Hulatt CJ, Wijffels RH, Kiron V (2019) Growth and LC-PUFA production of the cold-adapted microalga Koliella antarctica in photobioreactors. J Appl Phycol 31:981–997
Torzillo G, Sacchi A, Materassi R, Richmond A (1991) Effect of temperature on yield and night biomass loss in Spirulina platensis grown outdoor in tubular photobioreactor. J Appl Phycol 3:103–109
Vadiveloo A, Moheimani N, Alghamedi R, Cosgrove J, Alameh K, Parlevliet D (2016) Sustainable cultivation of microalgae by an insulated glazed glass plate photobioreactor. Biotechnol J 11:363–374
Vo HNP, Ngo HH, Guo W, Nguyen TMH, Liu Y, Liu Y, Nguyen DD, Chang SW (2018) A critical review on designs and applications of microalgae-based photobioreactors for pollutants treatment. Sci Total Environ
Vree JH, Bosma R, Janssen M, Barbosa MJ, Wijffels RH (2015) Comparison of four outdoor pilot-scale photobioreactors. Biotechnol Biofuels 8:215
Yang Z, Cheng J, Xu X, Zhou J, Cen K (2016) Enhanced solution velocity between dark and light areas with horizontal tubes and triangular prism baffles to improve microalgal growth in a flat-panel photo-bioreactor. Bioresour Technol 211:519–526
Acknowledgments
Murdoch University is acknowledged for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nwoba, E.G., Parlevliet, D.A., Laird, D.W. et al. Does growing Nannochloropsis sp. in innovative flat plate photobioreactors result in changes to fatty acid and protein composition?. J Appl Phycol 32, 3619–3629 (2020). https://doi.org/10.1007/s10811-020-02227-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02227-9