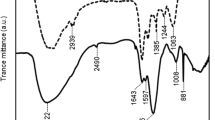
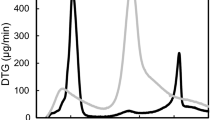
Abstract
Extracellular polysaccharide (EPS) can be distinguished into soluble or bound types and significantly contributes to colony formation in Microcystis. Depending on the binding strength with cells, the bilayer structure of bound EPS contains loosely or tightly bound EPS (LB-EPS or TB-EPS) and their roles in shaping the size and tightness of Microcystis colonies deserve further investigation. In this study, the influences of two types of bound EPS on the size and tightness of Microcystis colonies were investigated after a series of pretreatment to obtain LB-EPS retaining or stripped samples. Results showed that cells with LB-EPS formed large and loose colonies. Furthermore, the ratios of LB-EPS to TB-EPS, which indicate the size and tightness of the colonies, were higher in the retaining groups than in the stripped groups. Our findings also provide evidence that calcium enrichment is conducive to colony formation in Microcystis. This study provides new insights into the formation and enlargement of Microcystis colonies, which contributes to a better understanding on the role of EPS in Microcystis aggregation and morphology changes.






Similar content being viewed by others
References
Aktas TS, Takeda F, Maruo C, Chiba N, Nishimura O (2012) A comparison of zeta potentials and coagulation behaviors of cyanobacteria and algae. Desalin Water Treat 48:294–301
Bi X, Zhang S, Dai W, Xing K, Yang F (2013) Effects of lead (II) on the extracellular polysaccharide (EPS) production and colony formation of cultured Microcystis aeruginosa. Water Sci Technol 67:803–809
Duan Z, Tan X, Parajuli K, Upadhyay S, Zhang D, Shu X, Liu Q (2018) Colony formation in two Microcystis morphotypes: effects of temperature and nutrient availability. Harmful Algae 72:14–24
Gan N, Xiao Y, Zhu L, Wu Z, Liu J, Hu C, Song L (2012) The role of microcystins in maintaining colonies of bloom-forming Microcystis spp. Environ Microbiol 14:730–742
Hadjoudja S, Deluchat V, Baudu M (2010) Cell surface characterisation of Microcystis aeruginosa, and Chlorella vulgaris. J Colloid Interface Sci 342:293–299
Harke MJ, Steffen MM, Gobler CJ, Otten TG, Wilhelm SW, Wood SA, Paerl HW (2016) A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 54:4–20
Kessel M, Eloff JN (1975) The ultrastructure and development of the colonial sheath of Microcystis marginata. Arch Microbiol 106:209–214
Li X, Yang S (2007) Influence of loosely bound extracellular polymeric substances (EPS) on the flocculation, sedimentation and dewaterability of activated sludge. Water Res 41:1022–1030
Li H, Wen Y, Cao A, Huang J, Zhou Q, Somasundaran P (2012) The influence of additives (Ca2+, Al3+, and Fe3+) on the interaction energy and loosely bound extracellular polymeric substances (EPS) of activated sludge and their flocculation mechanisms. Bioresour Technol 114:188–194
Li L, Zhu W, Wang T, Luo Y, Chen F, Tan X (2013a) Effect of fluid motion on colony formation in Microcystis aeruginosa. Water Sci Eng 6:106–116
Li M, Zhu W, Gao L, Lu L (2013b) Changes in extracellular polysaccharide content and morphology of Microcystis aeruginosa at different specific growth rates. J Appl Phycol 25:1023–1030
Li M, Zhu W, Gao L (2014) Analysis of cell concentration, volume concentration, and colony size of Microcystis via laser particle analyzer. Environ Manag 53:947–958
Li M, Zhu W, Guo L, Hu J, Chen H, Xiao M (2016) To increase size or decrease density? Different Microcystis species has different choice to form blooms. Sci Rep 6:37056
Liu H, Fang H (2002) Characterization of electrostatic binding sites of extracellular polymers by linear programming analysis of titration data. Biotechnol Bioeng 80:806–811
Lürling M, Eshetu F, Faassen E, Kosten S, Huszar V (2013) Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshw Biol 58:552–559
Paerl H, Xu H, McCarthy M, Zhu G, Qin B, Li Y, Gardner W (2011) Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): the need for a dual nutrient (N & P) management strategy. Water Res 45:1973–1983
Qin B, Yang G, Ma J, Deng J, Li W, Wu T, Zhang Y (2016) Dynamics of variability and mechanism of harmful cyanobacteria bloom in Lake Taihu, China. Chin Sci Bull 61:759–770
Qu F, Liang H, Wang Z, Wang H, Yu H, Li G (2012) Ultrafiltration membrane fouling by extracellular organic matters (EOM) of Microcystis aeruginosa in stationary phase: influences of interfacial characteristics of foulants and fouling mechanisms. Water Res 46:1490–1500
Sato M, Amano Y, Machida M, Imazeki F (2017) Colony formation of highly dispersed Microcystis aeruginosa, by controlling extracellular polysaccharides and calcium ion concentrations in aquatic solution. Limnology 18:111–119
Shen H, Niu Y, Xie P, Tao M, Yang X (2011) Morphological and physiological changes in Microcystis aeruginosa as a result of interactions with heterotrophic bacteria. Freshw Biol 56:1065–1080
Sheng G, Yu H, Li X (2010) Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: a review. Biotechnol Adv 28:882–894
Stanier R, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Tang J, Wu Y, Esquivel-Elizondo S, Sørensen JS, Rittmann EB (2018) How microbial aggregates protect against nanoparticle toxicity. Trends Biotechnol 36:1171–1182
Visser PM, Passarge J, Mur LR (1997) Modelling vertical migration of the cyanobacterium Microcystis. Hydrobiologia 349:99–109
Vogelaar JCT, De Keizer A, Spijker S, Lettinga G (2005) Bioflocculation of mesophilic and thermophilic activated sludge. Water Res 39:37–46
Wang Y, Zhao J, Li J, Li S, Zhang L, Wu M (2011) Effects of calcium levels on colonial aggregation and buoyancy of Microcystis aeruginosa. Curr Microbiol 62:679–683
Wu Z, Song L (2008) Physiological comparison between colonial and unicellular forms of Microcystis aeruginosa Kütz (Cyanobacteria). Phycologia 47:98–104
Xiao M, Willis A, Burford MA, Li M (2017) A meta-analysis comparing cell-division and cell-adhesion in Microcystis colony formation. Harmful Algae 67:85–91
Xu H, He P, Wang G, Shao L (2010) Three-dimensional excitation emission matrix fluorescence spectroscopy and gel-permeating chromatography to characterize extracellular polymeric substances in aerobic granulation. Water Sci Technol 61:2931–2942
Xu H, Cai H, Yu G, Jiang H (2013) Insights into extracellular polymeric substances of cyanobacterium Microcystis aeruginosa using fractionation procedure and parallel factor analysis. Water Res 47:2005–2014
Xu H, Jiang H, Yu G, Yang L (2014) Towards understanding the role of extracellular polymeric substances in cyanobacterial Microcystis, aggregation and mucilaginous bloom formation. Chemosphere 117:815–822
Xu F, Zhu W, Xiao M, Li M (2016) Interspecific variation in extracellular polysaccharide content and colony formation of Microcystis spp. cultured under different light intensities and temperatures. J Appl Phycol 28:1533–1541
Yamamoto Y, Nakahara H (2009) Seasonal variations in the morphology of bloom-forming cyanobacteria in a eutrophic pond. Limnology 10:185–193
Yamamoto Y, Shiah F, Chen Y (2011) Importance of large colony formation in bloom-forming cyanobacteria to dominate in eutrophic ponds. Ann Limnol Int J Lim 47:167–173
Yang Z, Kong F (2012) Formation of large colonies: a defense mechanism of Microcystis aeruginosa under continuous grazing pressure by flagellate Ochromonas sp. J Limnol 71:e5
Yang Z, Kong F, Shi X, Cao H (2006) Morphological response of Microcystis aeruginosa to grazing by different sorts of zooplankton. Hydrobiologia 563:225–230
Yang Z, Kong F, Shi X, Zhang M, Xing P, Cao H (2008) Changes in the morphology and polysaccharide content of Microcystis aeruginosa (Cyanobacteria) during flagellate grazing. J Phycol 44:716–720
Yang Z, Geng L, Wang W, Zhang J (2012) Combined effects of temperature, light intensity, and nitrogen concentration on the growth and polysaccharide content of Microcystis aeruginosa in batch culture. Biochem Syst Ecol 41:130–135
Ye H, Yuan X, Ge M, Li J, Sun H (2010) Water chemistry characteristics and controlling factors in the northern rivers in the Taihu Basin. Ecol Environ Sci 19:23–27
Yu G, He P, Shao L (2009) Characteristics of extracellular polymeric substances (EPS) fractions from excess sludges and their effects on bioflocculability. Bioresour Technol 100:3193–3198
Funding
This study was sponsored by the National Natural Science Foundation of China (31470507), the Fundamental Research Funds for the Central Universities (2019B14014), and PAPD, the National Water Pollution Control and Treatment Science and Technology Major Project (2017ZX07603).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 187 kb)
Rights and permissions
About this article
Cite this article
Tan, X., Shu, X., Duan, Z. et al. Two types of bound extracellular polysaccharides and their roles in shaping the size and tightness of Microcystis colonies. J Appl Phycol 32, 255–262 (2020). https://doi.org/10.1007/s10811-019-01937-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01937-z