Abstract
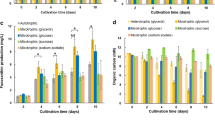
Open ponds are the preferred cultivation system for large-scale microalgal biomass production. To be more sustainable, commercial scale biomass production should rely on seawater, as freshwater is a limiting resource, especially in places with high irradiance. If seawater is used for both pond fill and evaporative volume makeup, salinity of the growth media will rise over time. It is not possible for any species to achieve optimum growth over the whole saline spectrum (from seawater salinity level up to salt saturation state). In this study, we investigated the effects of gradual salinity increase (between 35 and 233 ppt) on biomass productivity and biochemical composition (lipid and carbohydrate) of six marine, two halotolerant, and a halophilic microalgae. A gradual and slow stepped salinity increase was found to expand the salinity tolerance range of tested species. A gradual reduction in biomass productivity and maximum photochemical efficiency was observed as a consequence of increased salinity in all tested species. Among the marine microalgae, Tetraselmis showed highest biomass productivity (32 mg L−1 day−1) with widest salinity tolerance range (35 to 109 ppt). Halotolerant Amphora and Navicula were able to grow from 35 ppt to 129 ppt salinity. Halophilic Dunaliella was the only species capable of growing between 35 and 233 ppt and showed highest lipid content (56.2%) among all tested species. This study showed that it should be possible to maintain high biomass in open outdoor cultivation utilizing seawater by growing Tetraselmis, Amphora, and Dunaliella one after another as salinity increases in the cultivation system.



Similar content being viewed by others
References
Abiusi F, Sampietro G, Marturano G, Biondi N, Rodolfi L, D'Ottavio M, Tredici MR (2014) Growth, photosynthetic efficiency, and biochemical composition of Tetraselmis suecica F&M-M33 grown with LEDs of different colors. Biotechnol Bioeng 111:956–964
Al-Hasan R, Ghannoum M, Sallal A, Abu-Elteen K, Radwan S (1987) Correlative changes of growth, pigmentation and lipid composition of Dunaliella salina in response to halostress. Microbiology 133:2607–2616
Alkhamis Y, Qin JG (2013) Cultivation of Isochrysis galbana in phototrophic, heterotrophic, and mixotrophic conditions. BioMed Res Int 2013:983456
Allakhverdiev SI, Sakamoto A, Nishiyama Y, Inaba M, Murata N (2000) Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol 123:1047–1056
Anning T, Harris G, Geider R (2001) Thermal acclimation in the marine diatom Chaetoceros calcitrans (Bacillariophyceae). Eur J Phycol 36:233–241
Bartley ML, Boeing WJ, Corcoran AA, Holguin FO, Schaub T (2013) Effects of salinity on growth and lipid accumulation of biofuel microalga Nannochloropsis salina and invading organisms. Biomass Bioenergy 54:83–88
Bartual A, Gálvez JA (2003) Short-and long-term effects of irradiance and CO2 availability on carbon fixation by two marine diatoms. Can J Bot 81:191–200
Ben-Amotz A, Tornabene TG, Thomas WH (1985) Chemical profile of selected species of microalgae with emphasis on lipids. J Phycol 21:72–81
Benemann JR (1992) Microalgae aquaculture feeds. J Appl Phycol 4:233–245
Bhola VK, Swalaha FM, Nasr M, Kumari S, Bux F (2016) Physiological responses of carbon-sequestering microalgae to elevated carbon regimes. Eur J Phycol 51:401–412
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Borowitzka MA (1997) Microalgae for aquaculture: opportunities and constraints. J Appl Phycol 9:393–401
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Borowitzka MA, Moheimani NR (2013) Sustainable biofuels from algae. Mitig Adapt Strat Glob Chang 18:13–25
Boruff BJ, Moheimani NR, Borowitzka MA (2015) Identifying locations for large-scale microalgae cultivation in Western Australia: a GIS approach. Appl Energy 149:379–391
Brown A, Borowitzka LJ (1979) Halotolerance of Dunaliella. In: Levendowsky M, Hunter SH (eds) Biochemistry and physiology of protozoa. Academic Press, New York, pp 139–190
Cooksey K, Chansang H (1976) Isolation and physiological studies on three isolates of Amphora (Bacillariophyceae). J Phycol 12:455–460
Cosgrove JJ, Borowitzka MA (2010) Chlorophyll fluorescence terminology: an introduction. In: Suggett DJ, Borowitzka MA, Prášil O (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Springer, Dordrecht, pp 1–17
Dao LH, Beardall J (2016) Effects of lead on growth, photosynthetic characteristics and production of reactive oxygen species of two freshwater green algae. Chemosphere 147:420–429
Doan QC, Moheimani NR, Mastrangelo AJ, Lewis DM (2012) Microalgal biomass for bioethanol fermentation: implications for hypersaline systems with an industrial focus. Biomass Bioenergy 46:79–88
Fabregas J, Abalde J, Herrero C, Cabezas B, Veiga M (1984) Growth of the marine microalga Tetraselmis suecica in batch cultures with different salinities and nutrient concentrations. Aquaculture 42:207–215
Fon Sing S (2010) Strain selection and outdoor cultivation of halophilic microalgae with potential for large-scale biodiesel production. PhD Thesis, Murdoch University, Western Australia
Fon-Sing S, Borowitzka MA (2016) Isolation and screening of euryhaline Tetraselmis spp. suitable for large-scale outdoor culture in hypersaline media for biofuels. J Appl Phycol 28:1–14
Fon Sing S, Isdepsky A, Borowitzka MA, Lewis DM (2014) Pilot-scale continuous recycling of growth medium for the mass culture of a halotolerant Tetraselmis sp. in raceway ponds under increasing salinity: a novel protocol for commercial microalgal biomass production. Bioresour Technol 161:47–54
Frank G, Wegmann K (1974) Physiology and biochemistry of glycerol biosynthesis in Dunaliella. Biol Zentralbl 93:707
Fujii S, Nishimoto N, Notoya A, Hellebust JA (1995) Growth and osmoregulation of Chaetoceros muelleri in relation to salinity. Plant Cell Physiol 36:759–764
Gibson JAE, Roberts D, Van de Vijver B (2006) Salinity control of the distribution of diatoms in lakes of the Bunger Hills, East Antarctica. Polar Biol 29:694–704
Griffiths MJ, Harrison ST (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 215:493–507
Gu N, Lin Q, Li G, Tan Y, Huang L, Lin J (2012) Effect of salinity on growth, biochemical composition, and lipid productivity of Nannochloropsis oculata CS 179. Engi Life Sci 12:631–637
Guillard RR (1975) Culture of phytoplankton for feeding marine invertebrates. In: Chanley MH, Smith WL (eds) Culture of marine invertebrate animals. Springer, Boston, pp 29–60
Guillard RR, Ryther JH (1962) Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Hart BT, Bailey P, Edwards R, Hortle K, James K, McMahon A, Meredith C, Swadling K (1991) A review of the salt sensitivity of the Australian freshwater biota. Hydrobiologia 210:105–144
Hellebust JA (1976) Effect of salinity on photosynthesis and mannitol synthesis in the green flagellate Platymonas suecica. Can J Bot 54:1735–1741
Hellebust JA (1985) Mechanisms of response to salinity in halotolerant microalgae. Plant Soil 89:69–81
Hillebrand H, Dürselen CD, Kirschtel D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J Phycol 35:403–424
Iluz D, Dubinsky Z (2013) Quantum yields in aquatic photosynthesis. In: Dubinsky Z (ed) Photosynthesis. InTech, Rijeka, pp 135–158
Indrayani I (2017) Isolation and characterization of microalgae with commercial potential. PhD Thesis, Murdoch University, Western Australia 214 pp
Isdepsky A (2015) Saline microalgae for biofuels: Outdoor culture from small-scale to pilot scale. PhD Thesis Murdoch University, Western Australia 240 pp
Ishika T, Moheimani NR, Bahri PA (2017) Sustainable saline microalgae co-cultivation for biofuel production: a critical review. Renew Sustain Energy Rev 78:356–368
Kan G, Shi C, Wang X, Xie Q, Wang M, Wang X, Miao J (2012) Acclimatory responses to high-salt stress in Chlamydomonas (Chlorophyta, Chlorophyceae) from Antarctica. Acta Oceanol Sinica 31:116–124
Kaplan D, Cohen Z, Abeliovich A (1986) Optimal growth conditions for Isochrysis galbana. Biomass 9:37–48
Kates M, Volcani B (1966) Lipid components of diatoms. Biochim Biophys Acta Lipids Lipid Metabol 116:264–278
Kirst G (1990) Salinity tolerance of eukaryotic marine algae. Annu Rev Plant Biol 41:21–53
Kirst G, Bisson M (1979) Regulation of turgor pressure in marine algae: ions and low-molecular-weight organic compounds. Aust J Plant Physiol 6:539–556
Kochert G (1978) Carbohydrate determination by the phenol-sulfuric acid method. In: Helebust JA, Craig JS (eds) Handbook of phycological methods. Cambridge University Press, Cambridge, pp 95–97
Kromkamp J, Peene J (1999) Estimation of phytoplankton photosynthesis and nutrient limitation in the eastern Scheldt estuary using variable fluorescence. Aquat Ecol 33:101–104
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14:217–232
McLachlan J (1961) The effect of salinity on growth and chlorophyll content in representative classes of unicellular marine algae. Can J Microbiol 7:399–406
Mercado JM, del Pilar Sánchez-Saavedra M, Correa-Reyes G, Lubián L, Montero O, Figueroa FL (2004) Blue light effect on growth, light absorption characteristics and photosynthesis of five benthic diatom strains. Aquat Bot 78:265–277
Mercz TI (1994) A study of high lipid yielding microalgae with potential for large-scale production of lipids and polyunsaturated fatty acids. PhD Thesis, Murdoch University, Westerm Australia 278 pp
Mohamad IB, Usman D (2013) Standardization and its effects on K-means clustering algorithm. Res J Appl Sci Engi Technol 6:3299–3303
Moheimani NR (2013a) Inorganic carbon and pH effect on growth and lipid productivity of Tetraselmis suecica and Chlorella sp (Chlorophyta) grown outdoors in bag photobioreactors. J Appl Phycol 25:387–398
Moheimani NR (2013b) Long-term outdoor growth and lipid productivity of Tetraselmis suecica, Dunaliella tertiolecta and Chlorella sp (Chlorophyta) in bag photobioreactors. J Appl Phycol 25:167–176
Moheimani NR, Borowitzka MA (2011) Increased CO2 and the effect of pH on growth and calcification of Pleurochrysis carterae and Emiliania huxleyi (Haptophyta) in semicontinuous cultures. Appl Microbiol Biotechnol 90:1399–1407
Moheimani NR, Borowitzka MA, Isdepsky A, Fon Sing S (2013) Standard methods for measuring growth of algae and their composition. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer, Dordrecht, pp 265–284
Pal D, Khozin-Goldberg I, Cohen Z, Boussiba S (2011) The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl Microbiol Biotechnol 90:1429–1441
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648
Ramanna L, Guldhe A, Rawat I, Bux F (2014) The optimization of biomass and lipid yields of Chlorella sorokiniana when using wastewater supplemented with different nitrogen sources. Bioresour Technol 168:127–135
Renaud SM, Parry DL (1994) Microalgae for use in tropical aquaculture II: Effect of salinity on growth, gross chemical composition and fatty acid composition of three species of marine microalgae. J Appl Phycol 6:347–356
Santos MMD, Moreno-Garrido I, Gonçalves F, Soares AM, Ribeiro R (2002) An in situ bioassay for estuarine environments using the microalga Phaeodactylum tricornutum. Environ Toxicol Chem 21:567–574
Simionato D, Sforza E, Corteggiani Carpinelli E, Bertucco A, Giacometti GM, Morosinotto T (2011) Acclimation of Nannochloropsis gaditana to different illumination regimes: effects on lipids accumulation. Bioresour Technol 102:6026–6032
Smith CM, Berry JA (1986) Recovery of photosynthesis after exposure of intertidal algae to osmotic and temperature stresses: comparative studies of species with differing distributional limits. Oecologia 70:6–12
Strizh I, Popova L, Balnokin YV (2004) Physiological aspects of adaptation of the marine microalga Tetraselmis (Platymonas) viridis to various medium salinity. Russ J Plant Physiol 51:176–182
Takagi M, Karseno, Yoshida T (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101:223–226
Vazquez-Duhalt R, Arredondo-Vega BO (1991) Haloadaptation of the green alga Botryococcus braunii (Race A). Phytochemistry 30:2919–2925
White S, Anandraj A, Trois C (2013) The effect of landfill leachate on hydrogen production in Chlamydomonas reinhardtii as monitored by PAM fluorometry. Int J Hydrog Energy 38:14214–14222
Yang J, Xu M, Zhang X, Hu Q, Sommerfeld M, Chen Y (2011) Life-cycle analysis on biodiesel production from microalgae: water footprint and nutrients balance. Bioresour Technol 102:159–165
Acknowledgements
We are thankful to the following undergraduate students: Emma Moulton and Jack Weatherhead, School of Veterinary and Life Sciences, Murdoch University, WA, for their assistance in extracting lipids and carbohydrates.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 68 kb)
Rights and permissions
About this article
Cite this article
Ishika, T., Bahri, P.A., Laird, D.W. et al. The effect of gradual increase in salinity on the biomass productivity and biochemical composition of several marine, halotolerant, and halophilic microalgae. J Appl Phycol 30, 1453–1464 (2018). https://doi.org/10.1007/s10811-017-1377-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1377-y