Abstract
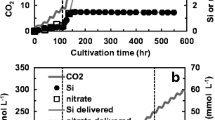
The photosynthetic diatom Cyclotella sp. extrudes chitin nanofibers following cell division. This diatom requires silicon for cell wall biosynthesis and division, as well as nitrogen for biosynthesis of intracellular material and extracellular chitin, an N-acetyl glucosamine biopolymer. The initial nitrogen/silicon molar ratio was the critical parameter for assessing the limits of nitrogen delivery on cell number and chitin production during batch cultivation of Cyclotella in a bubble column photobioreactor under silicon-limited growth conditions, using nitrate as the nitrogen source. The peak rate of volumetric chitin production increased linearly, from 3.0 to 46 mg chitin L−1 day−1, with increasing N/Si ratio over the range studied (0.82 to 8.6 mol N mol−1 Si). However, the cell number yield and the chitin yield per cell increased asymptotically with increasing N/Si ratio, achieving a final cell number yield of 5.3 × 109 ± 2.6 × 108 cells mol−1 Si and chitin yield of 28.7 ± 1.2 mg chitin per 109 cells (1.0 S.E.). An N/Si ratio of at least 4.0 mol N mol−1 Si achieved 90% of the asymptotic chitin yield. This study has shown that scalable cultivation systems for maximizing chitin nanofiber production will require delivery of both silicon and optimal nitrogen under silicon-limiting growth conditions to promote cell division and subsequent chitin formation.





Similar content being viewed by others
References
Azuma K, Ifuku S, Osaki T, Okamoto Y, Minami S (2014) Preparation and biomedical applications of chitin and chitosan nanofibers. J Biomed Nanotechnol 10:2891–2920
Chiriboga OG, Rorrer GL (2017) Control of chitin nanofiber production by the lipid-producing diatom Cyclotella sp. through fed-batch addition of dissolved silicon and nitrate in a bubble-column photobioreactor. Biotechnol Prog 33:407–415
Ding F, Deng H, Du Y, Shi X, Wang Q (2014) Emerging chitin and chitosan nanofibrous materials for biomedical applications. Nanoscale 6:9477–9493
Gügi B, Le Costaouec T, Burel C, Lerouge P, Helbert W, Bardor M (2015) Diatom-specific oligosaccharide and polysaccharide structures help to unravel biosynthetic capabilities in diatoms. Mar Drugs 13:5993–6018
Habibi Y, Lucia LA (2012) Chitin nanofibers as building blocks for advanced materials. In: Habibi Y, Lucia LA (eds) Polysaccharide Building Blocks: A Sustainable Approach to the Development of Renewable Biomaterials. John Wiley & Sons, Inc, Hoboken, pp 227–245
Herth W (1979) The site of β-chitin fibril formation in centric diatoms II. The chitin-forming cytoplasmic structures. J Ultrastruct Res 68:16–27
Herth W, Barthlott W (1979) The site of β-chitin fibril formation in centric diatoms I. Pores and fibril formation. J Ultrastruct Res 68:6–15
Herth W, Zugenmaier P (1977) Ultrastructure of the chitin fibrils of the centric diatom Cyclotella cryptica. J Ultrastruct Res 61:230–239
Ifuku S, Saimoto H (2012) Chitin nanofibers: preparations, modifications, and applications. Nanoscale 4:3308–3318
Jayakumar R, Prabaharan M, Nair SV, Tamura H (2010) Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol Adv 28:142–150
Jeffryes C, Gutu T, Jiao J, Rorrer GL (2008) Two-stage photobioreactor process for the metabolic insertion of nanostructured germanium into the silica microstructure of the diatom Pinnularia sp. Mater Sci Eng C 28:107–118
Kamatani A, Riley JP (1979) Rate of dissolution of diatom silica walls in seawater. Mar Biol 55:29–35
Kato K, Kitano Y (1968) Solubility and dissolution rate of amorphous silica in distilled and sea water at 20 °C. J Oceanogr Soc Jpn 24:147–152
Krauskopf KB (1956) Dissolution and precipitation of silica at low temperatures. Geochim Cosmochim Acta 10:1–26
Martin-Jézéquel V, Hildebrand M, Brzezinski MA (2000) Silicon metabolism in diatoms: implications for growth. J Phycol 36:821–840
McLachlan J, McInnes G, Falk M (1965) Studies on the chitin (chitin: poly-N-acetylglucosamine) fibers of the diatom Thalassiosira fluviatilis Hustedt. I Production and isolation of chitin fibers. Can J Bot 43:707–713
Merzendorfer H (2011) The cellular basis of chitin synthesis in fungi and insects: common principles and differences. Eur J Cell Biol 90:759–769
Obata T, Fernie AR, Nunes-Nesi A (2013) The central carbon and energy metabolism of marine diatoms. Metabolites 3:325–346
Ogawa Y, Kimura S, Wada M (2011) Electron diffraction and high-resolution imaging on highly-crystalline β-chitin microfibril. J Struct Biol 176:83–90
Ozkan A, Rorrer GL (2017a) Effects of light intensity on the selectivity of lipid and chitin nanofiber production during photobioreactor cultivation of the marine diatom Cyclotella sp. Algal Res 25:216–227
Ozkan A, Rorrer GL (2017b) Lipid and chitin nanofiber production during cultivation of the marine diatom Cyclotella sp. to high cell density with multistage addition of silicon and nitrate. J Appl Phycol 29:1811–1818
Ozkan A, Rorrer GL (2017c) Effects of CO2 delivery on fatty acid and chitin nanofiber production during photobioreactor cultivation of the marine diatom Cyclotella sp. Algal Res 26:422–430
Rolandi M, Rolandi R (2014) Self-assembled chitin nanofibers and applications. Adv Colloid Interf Sci 207:216–222
Rorrer GL, Antonio Torres J, Durst R, Kelly C, Gale D, Maddux B, Ozkan A (2016) The potential of a diatom-based photosynthetic biorefinery for biofuels and valued co-products. Curr Biotechnol 5:237–248
Smith SR, Abbriano RM, Hildebrand M (2012) Comparative analysis of diatom genomes reveals substantial differences in the organization of carbon partitioning pathways. Algal Res 1:2–16
Takabayashi M, Wilkerson FP, Robertson D (2005) Response of glutamine synthetase gene transcription and enzyme activity to external nitrogen sources in the diatom Skeletonema costatum (Bacillariophyceae). J Phycol 41:84–94
Traller JC, Cokus SJ, Lopez DA, Gaidarenko O, Smith SR, McCrow JP, Gallaher SD, Podell S, Thompson M, Cook O, Morselli M, Jaroszewicz A, Allen EE, Allen AE, Merchant SS, Pellegrini M, Hildebrand M (2016) Genome and methylome of the oleaginous diatom Cyclotella cryptica reveal genetic flexibility toward a high lipid phenotype. Biotechnol Biofuels 9:258
Acknowledgements
This work was supported by the US National Science Foundation (NSF), Emerging Frontiers for Research and Innovation program (EFRI), under award number 1240488.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiriboga, O., Rorrer, G.L. Effects of nitrogen delivery on chitin nanofiber production during batch cultivation of the diatom Cyclotella sp. in a bubble column photobioreactor. J Appl Phycol 30, 1575–1581 (2018). https://doi.org/10.1007/s10811-017-1368-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1368-z