Abstract
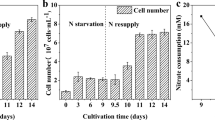
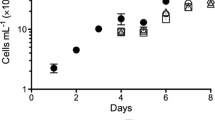
Induction of oil accumulation in algae for biofuel production is often achieved by withholding nitrogen. However, withholding nitrogen often reduces total biomass yield. In this report, it is demonstrated that Chlorella sorokiniana will not only accumulate substantial quantities of neutral lipids when grown in the absence of nitrogen but will also exhibit unimpeded growth rates for up to 2 weeks. To determine the physiological basis for the observed increase in oil and biomass accumulation, we compared photosynthetic and respiration rates and chlorophyll, lipid, and total energy content under ammonia replete and deplete conditions. Under N-depleted growth conditions, there was a 64 % increase in total energy density and a ∼20-fold increase in oil accumulation relative to N-replete growth leading to a 1.6-fold greater total energy yield in N-depleted than in N-replete cultures. We propose that the higher energy accumulation in N-depleted cultures is due to enhanced photosynthetic energy capture and conversion associated with reduced chlorophyll levels and reduced self-shading as well as a shift in metabolism leading to the accumulation of oils.





Similar content being viewed by others
References
Berges JA, Charlebois DO, Mauzerall DC, Falkowski PG (1996) Differential effects of nitrogen limitation on photosynthetic efficiency of photosystems I and II in microalgae. Plant Physiol 110:689–696
Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2012) The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour Technol 124:217–226
Cakmak T, Angun P, Demiray YE, Ozkan AD, Elibol Z, Tekinay T (2012) Differential effects of nitrogen and sulfur deprivation on growth and biodiesel feedstock production of Chlamydomonas reinhardtii. Biotechnol Bioeng 109:1947–1957
Carvalho AP, Silva SO, Baptista JM, Malcata FX (2011) Light requirements in microalgal photobioreactors: an overview of biophotonic aspects. Appl Microbiol Biotechnol 89:1275–1288
de Loura IC, Dubacq JP, Thomas JC (1987) The effects of nitrogen deficiency on pigments and lipids of cyanobacteria. Plant Physiol 83:838–843
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Holguin F, Schaub T (2013) Characterization of microalgal lipid feedstocks by direct infusion FT-ICR mass spectrometry. Algal Res 2:43–50
Huerlimann R, de Nys R, Heimann K (2010) Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale-up production. Biotechnol Bioeng 107:245–257
Li T, Zheng Y, Yu L, Chen S (2013) High productivity cultivation of a heat-resistant microalga Chlorella sorokiniana for biofuel production. Bioresour Technol 131:60–67
Lizzul AM, Hellier P, Purton S, Baganz F, Ladommatos N, Campos L (2014) Combined remediation and lipid production using Chlorella sorokiniana grown on wastewater and exhaust gases. Bioresour Technol 151:12–18
Lourenco SO, Barbarino E (1998) Distribution of intrecellular nitrogen in marine microalgae: basis for the calculation of specific nitrogen-to-protein conversion factors. J Phycol 34:798–811
Lu C, Zhang J, Zhang Q, Li L, Kuang T (2001) Modification of photosystem II photochemistry in nitrogen deficient maize and wheat proteins. J Plant Physiol 158:1423–1430
Lv JM, Cheng LH, Xu XH, Zhang L, Chen HL (2010) Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour Technol 101:6797–6804
Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Bioresour Technol 131:60–67
Murphy TE, Berberoglu H (2012) Effect of algae pigmentation on photobioreactor productivity and scale-up: a light transfer perspective. J Quant Spectrosc Radiat Transf 112:2826–2834
Neidhardt J, Benemann JR, Zhang L, Melis A (1998) Photosystem-II repair and chloroplast recovery from irradiance stress: relationship between chronic photoinhibition, light-harvesting chlorophyll antenna size and photosynthetic productivity in Dunaliella salina (green algae). Photosynth Res 56:175–184
Perrine Z, Negi S, Sayre RT (2012) Optimization of photosynthetic light energy utilization by microalgae. Algal Res 1:134–142
Pruvost J, Van Vooren G, Le Gouic B, Couzinet-Mossion A, Legrand J (2011) Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour Technol 102:150–158
Ramanna L, Guldhe A, Rawat I, Bux F (2014) The optimization of biomass and lipid yields of Chlorella sorokiniana when using wastewater supplemented with different nitrogen sources. Bioresour Technol 168:127–135
Rosenberg JN, Kobayashi N, Barnes A, Noel EA, Betenbaugh MJ, Oyler GA (2014) Comparative analyses of three Chlorella species in response to light and sugar reveal distinctive lipid accumulation patterns in the microalga C. sorokiniana. PLoS One 9:e92460
Sayed OH, Hegazy AK (1992) Inhibition of secondary carotenoid biosynthesis during degreening of Chlorella fusca (Chlorococcales, Chlorophyta) and implication for growth and survival. Cryptogam Algol 13:181–186
Sayre RT (2010) Microalgal biofuels; carbon capture and sequestration. Bioscience 60:722–727
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5:1532–1553
Siaut M, Cuine S, Cagnon C, Fessler B, Nguyen M, Carrier P, Beyly A, Beisson F, Triantaphylides C, Li-Beisson Y, Peltier G (2011) Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol 11:1–15
Simionato D, Block MA, La Rocca N, Jouhet J, Marechal E, Finazzi G, Morosinotto T (2013) Response of Nannochloropsis gaditana to nitrogen starvation includes a de novo biosynthesis of triacylglycerols, a decrease of chloroplast galactolipids and a reorganization of the photosynthetic apparatus. Eukaryot Cell 12:665–676
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Subramanian S, Barry AN, Pieris S, Sayre RT (2013) Comparative energetics and kinetics of autotrophic lipid and starch metabolism in chlorophytic microalgae: implications for biomass and biofuel production. Biotechnol Biofuels 6:150–162
Sueoka N (1960) Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardi. Proc Natl Acad Sci 46:83–91
Tamburic B, Guruprasad S, Radford DT, Szabo M, Lilley RM, Larkum AW, Franklin JB, Kramer DM, Blackburn SI, Raven JA, Schliep M, Ralph PJ (2014) The effect of diel temperature and light cycles on the growth of Nannochloropsis oculata in a photobioreactor matrix. PLoS One 9:e86047
Tang H, Chen M, Garcia ME, Abunasser N, Ng KY, Salley SO (2011) Culture of microalgae Chlorella minutissima for biodiesel feedstock production. Biotechnol Bioeng 108:2280–2287
Wang S-T, Pan Y-Y, Liu C-C, Chuang L-T, Chen C-NN (2011) Characterization of a green microalga UTEX 2219–4: effects of photosynthesis and osmotic stress on oil body formation. Bot Stud 52:305–312
Zhang YM, Chen H, He CL, Wang Q (2013) Nitrogen starvation induced oxidative stress in an oil-producing green alga Chlorella sorokiniana C3. PLoS One 8:e69225
Acknowledgments
We thank Paige Pardington for her help in assisting with the photobioreactors during this experiment. This work is supported by the U.S. Department of Energy under contract DE-EE0003046 awarded to the National Alliance for Advanced Biofuels and Bioproducts for RTS and TS and by the Center for Animal Health and Food Safety at New Mexico State University for TS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sangeeta Negi and Amanda N. Barry shared first author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 436 kb)
Rights and permissions
About this article
Cite this article
Negi, S., Barry, A.N., Friedland, N. et al. Impact of nitrogen limitation on biomass, photosynthesis, and lipid accumulation in Chlorella sorokiniana . J Appl Phycol 28, 803–812 (2016). https://doi.org/10.1007/s10811-015-0652-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0652-z