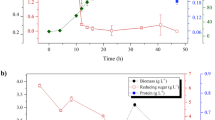
Abstract
In the present study, tolerance of ten microalgal strains isolated from wastewaters of different textile mills in relation to two metals and dyes was studied based on cell growth estimated spectrophotometrically. Three cyanobacterial strains that were found to tolerate both Cr(VI) and Co(II) along with the dyes reactive red 198 (RR 198) and crystal violet (CV) were investigated further for the concentration of various photosynthetic pigments and exopolymer production in the presence of the dyes and metals. All three tolerant species—Nostoc linckia HA-46, Myxosarcina spectabilis HP-43 and Gloeocapsa calcarea HP-45—showed a significantly higher concentration (P < 0.05) of various pigments when the medium was spiked with metals or dyes. Production of extracellular proteins and particularly extracellular polysaccharides by the tolerant strains increased significantly (P < 0.05) in the presence of metals. The effect of dyes was, however, not always statistically significant (P > 0.05). Production of hydrogen by these photoautotrophic microbes was moderate (19–28 nmol h−1 mg−1 dry wt). The best performing strain, N. linckia, when examined further for its hydrogen production potential in the presence of the two dyes and metals, showed significantly higher rates of hydrogen production in the presence of Cr, Co and RR 198. Its hydrogenase activity also followed the same trend. Immobilization of the microbe into alginate beads almost doubled the hydrogen production by the organism in the control as well as in the presence of suitable concentrations of the two metals (10 mg L−1) and dyes (50 mg L−1).

Similar content being viewed by others
References
Aguilera A, Amils R (2005) Tolerance to cadmium in Chlamydomonas sp. (Chlorophyta) strains isolated from an extreme acidic environment, the Tinto River (SW, Spain). Aq Toxicol 75:316–329
Arica MY, Bayramoglu G, Yilmaz M, Ince O, Bektas S, Genc O (2004) Biosorption of Hg2+, Cd2+, and Zn2+ by Ca-alginate and immobilized fungus Funalia trogii. J Hazard Mater 109:191–199
Arora NK, Khare E, Singh S, Maheshwari DK (2010) Effect of Al and heavy metals on enzymes of nitrogen metabolism of fast and slow growing rhizobia under explanta conditions. World J Microbiol Biotechnol 26:811–816
Banerjee M, Kumar A, Kumar HD (1989) Factors regulating nitrogenase activity and hydrogen evolution in Azolla–Anabaena symbiosis. Int J Hydrogen Energy 14:871–879
Benemann JR (1998) The technology of biohydrogen. In: Zaborsky O (ed) Bio Hydrogen. Plenum, New York, pp 19–30
Bennett A, Bogorad L (1971) Properties of subunits and aggregates of bluegreen algal biliproteins. Biochemistry 10:3625–3634
Coolidge FL (2000) Statistics. SAGE Pub, London, p 287
De Philippis R, Vinenzini M (1998) Extracellular polysaccharides from cyanobacteria and their possible applications. FEMS Microb Rev 22:151–175
Desikachary TV (1959) Cyanophyta. Indian Council of Agricultural Research, New Delhi, p 686
Foster PL (1982) Species association and metal contents of algae from rivers polluted with heavy metals. Freshwat Biol 12:17–39
Ghirardi ML, Kosourov S, Tsygankov A, Rubin A, Seibert M (2002) Cyclic photobiological algal H2-production. In: Proceedings of the 2002 U.S. DOE Hydrogen Program Review. NREL/CP-610-32405 p 1–12
Ghirardi ML, Dubini A, Yu J, Maness PC (2009) Photobiological hydrogen-producing systems. Chem Soc Rev 38:52–61
Goyal N, Jain SC, Banerjee UC (2003) Comparative studies on the microbial adsorption of heavy metals. Advan Environ Res 7:311–319
Greger M, Orgen E (1991) Direct and indirect effects of Cd2+ on photosynthesis in sugar beet (Beta vulgaris). Plant Physiol 83:129–135
Hansel A, Lindblad P (1998) Mini-review: towards optimization of cyanobacteria as biotechnologically relevant producers of molecular hydrogen, a clean and renewable energy source. App Environ Microbiol 50:153–160
Hardy RWF, Holsten RD, Jackson EK, Burns RC (1968) The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol 43:1185–1207
Heng LY, Jusoh K, Ling CHM, Idris M (2004) Toxicity of single and combination of lead and cadmium to the cyanobacterium Anabaena flos-aquae. Bull Environ Contam Toxicol 42:373–379
Horcsik Z, Olah V, Balogh A, Meszaros I, Simon L, Lakatos G (2006) Effect of chromium(VI) on growth, element and photosynthetic pigment composition of Chlorella pyrenoidosa. Acta Biologica Szegediensis 50:19–23
Hu TL, Wu SC (2001) Assessment of the effect of azo dye RP2B on the growth of a nitrogen fixing cyanobacterium—Anabaena sp. Bioresour Technol 77:93–95
Jensen A (1978) Chlorophylls and carotenoids. In: Hellebust JA, Craige JS (eds) Handbook of phycological methods, physiological and biochemical methods. Cambridge University Press, Cambridge, pp 59–70
Karube I, Ikemoto H, Kajiwara K, Tamiya E, Matsuoka H (1986) Photochemical energy conversion using immobilized blue-green algae. J Biotechnol 4:73–80
Katz SA, Salem H (1993) The toxicology of chromium with respect to its chemical speciation: a review. J Appl Toxicol 13:217–224
Kaushik BD (1987) Laboratory methods for blue-green algae. Associated Publishing Company, New Delhi, p 171
Kaushik A, Anjana K (2011) Biohydrogen production by Lyngbya perelegans: influence of physico-chemical environment. Biomass Bioenergy 35:1041–1045
Kiran B, Kaushik A, Kaushik CP (2008a) Metal-salt co-tolerance and metal removal by indigenous cyanobacterial strains. Process Biochem 43:598–604
Kiran B, Nisha R, Kaushik A (2008b) Chromium(VI) tolerance in two halotolerant strains of Nostoc. J Environ Biol 29:155–158
Kosourov NS, Seibert M (2009) Hydrogen photoproduction by nutrient-deprived Chlamydomonas reinhardtii cells immobilized within thin alginate films under aerobic and anaerobic conditions. Biotechnol Bioeng 102:50–58
Kosourov S, Tsygankov A, Seibert M, Ghirardi ML (2002) Sustained hydrogen photoproduction by Chlamydomonas reinhardtii: effects of culture parameters. Biotechn Bioeng 78:731–740
Kumar D, Kumar HD (1992) Hydrogen production by several cyanobacteria. Int J Hydrogen Energy 17:847–852
Leigh JS Jr, Dutton PL (1972) The primary electron acceptor in photosynthesis. Biochem Biophys Res Comm 46:414–421
Lowry OH, Rosebrough NJ, Larr AL, Randal RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
McKinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Miura Y, Matsuoka S, Miyamoto K, Satoh C (1992) Stably sustained hydrogen production with high molar yield through a combination of a marine green alga and a photosynthetic bacterium. Biosci Biotech Biochem 56:751–754
Mona S, Kaushik A, Kaushik CP (2011) Hydrogen production and metal-dye bioremoval by a Nostoc linckia strain isolated from textile mill oxidation pond. Bioresour Technol 102:3200–3205
Nieboer E, Jusys AA (1988) Biological chemistry of chromium. In: Nriagu JO, Nieboer (eds) Chromium in natural and human environments. Wiley, New York, pp 21–81
Pandey A, Pandey VD (2013) Evaluation of filamentous cyanobacteria for phycobiliproteins content and composition. Ind J Fund Appl Life Sci 3(2):222–227
Pearce CI, Lloyd JR, Guthric JT (2003) The removal of colour from textile wastewater using whole bacterial cell: a review. Dyes Pigments 58:179–196
Ramakrishnan M, Nagarajan S (2009) Utilization of waste biomass for the removal of basic dye from water. World Appl Sci J 5:114–121
Rao KK, Hall DO (1996) Hydrogen production by cyanobacteria: potential problems and prospects. J Mar Biotech 4:10–15
Salguero A, Benito M, Vigara J, Vega JM, Vilchez C, León R (2003) Carotenoids as protective response against oxidative damage in Dunaliella bardawil. Biomol Eng 20:249–253
Sarker S, Pandey KD, Kashyap AK (1992) Hydrogen photoproduction by filamentous non-heterocystous cyanobacterium Plectonema boryanna and simultaneous release of ammonia. Int J Hydrogen Energy 17:689–694
Seifter S, Dayton S, Novic B, Muntusylar E (1959) Estimation of glycogen with anthrone reagent. Arch Biochem 25:191–200
Shah V, Garg N, Madamwar D (2001) Ultrastructure of the fresh water cyanobacterium Anabaena variabilis SPU 003 and its application for oxygen-free hydrogen production. FEMS Microb Lett 194:71–75
Sharma M, Kaushik A, Somvir BK, Kamra A (2008) Sequestration of chromium by exopolysaccharides of Nostoc and Gloeocapsa from dilute aqueous solutions. J Hazard Mater 157:315–318
Sharma M, Rani N, Kamra A, Bala K, Kaushik A (2009) Growth, exopolymer production and metal bioremoval by Nostoc punctiforme in Na+ and Cr(VI) spiked medium. J Environ Res Dev 4:372–379
Shukla SP, Kumar AT, Tiwari DN, Mishra BP, Gupta GS (1994) Assessment of the effect of the toxicity of a textile dye on Nostoc muscorum ISU, a diazotrophic cyanobacterium. Environ Poll 84:23–25
Stainer RY, Dousoroff M, Omston IW (1971) General Microbiol. Macmillan, London, p 686
Stratton GW, Corke CT (1979) The effect of Ni on the growth, photosynthesis and nitrogenase activity of Anabaena inaequalis. Can J Microbiol 25:1094–1099
Takamura N, Kasai F, Watanabe MM (1989) Effects of Cu, Cd, and Zn on photosynthesis of freshwater benthic algae. J Appl Phycol 1:39–52
Vajpayee P, Tripathi RD, Rai UN, Ali MB, Singh SN (2000) Chromium accumulation reduces chlorophyll biosynthesis, nitrate reductase activity and protein content of Nymphaea alba. Chemosphere 41:1075–1082
Whitton BA (1970) Toxicity of heavy metals to Chlorophyta from running waters. Arch Microbiol 72:353–360
Yruela I, Alfonso M, Baron M, Picorel R (2000) Copper effect on the protein composition of photosystem II. Plant Physiol 110:551–557
Acknowledgements
The research was funded by a grant from University Grants Commission (UGC-SAP-DRS-II), New Delhi, and the authors also acknowledge the financial assistance from Ms. Mona Sharma by CSIR, New Delhi, in the form of Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mona, S., Kaushik, A. Screening metal-dye-tolerant photoautotrophic microbes from textile wastewaters for biohydrogen production. J Appl Phycol 27, 1185–1194 (2015). https://doi.org/10.1007/s10811-014-0396-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0396-1