Abstract
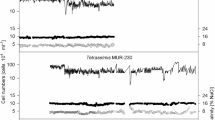
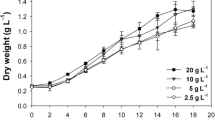
Microalgae cultivation systems can be divided broadly into open ponds and closed photobioreactors. This study investigated the growth and biomass productivity of the halophilic green alga Tetraselmis sp. MUR-233, grown outdoors in paddle wheel-driven open raceway ponds and in a tubular closed photobioreactor (Biocoil) at a salinity of 7 % NaCl (w/v) between mid-March and June 2010 (austral autumn/winter). Volumetric productivity in the Biocoil averaged 67 mg ash-free dry weight (AFDW) L−1 day−1 when the culture was grown without CO2 addition. This productivity was 86 % greater, although less stable, than that achieved in the open raceway pond (36 mg L−1 day−1) grown at the same time in the autumn period. The Tetraselmis culture in the open raceway pond could be maintained in semi-continuous culture for the whole experimental period of 3 months without an additional CO2 supply, whereas in the Biocoil, under the same conditions, reliable semi-continuous culture was only achievable for a period of 38 days. However, stable semi-continuous culture was achieved in the Biocoil by the addition of CO2 at a controlled pH of ~7.5. With CO2 addition, the volumetric biomass productivity in the Biocoil was 85 mg AFDW L−1 day−1 which was 5.5 times higher than the productivity achieved in the open raceway pond (15 mg AFDW L−1 day−1) with CO2 addition and 8 times higher compared to the productivity in the open raceway pond without CO2 addition (11 mg AFDW L−1 day−1), when cultures were grown in winter. The illuminated area productivities highlight an alternative story and showed that the open raceway pond had a three times higher productivity (3,000 mg AFDW m−2 day−1) compared to the Biocoil (850 mg AFDW m−2 day−1). Although significant differences were found between treatments and cultivation systems, the overall average lipid content for Tetraselmis sp. MUR-233 was 50 % in exponential phase during semi-continuous cultivation.



Similar content being viewed by others
References
Apt KE, Behrens PW (1999) Commercial developments in microalgal biotechnology. J Phycol 35:215–226
Benemann JR (1997) CO2 mitigation with microalgae systems. Energ Conv Manag 38:S475–S479
Bligh E, Dyer W (1959) A rapid method of total lipid extraction and purification. Can J Physiol Pharmacol 37:911–917
Borowitzka M (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Borowitzka MA, Moheimani NR (2013) Open pond culture systems. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer, Dordrecht, pp 133–152
Bosma R, van Zessen E, Reith JH, Tramper J, Wijffels RH (2007) Prediction of volumetric productivity of an outdoor photobioreactor. Biotech Bioeng 97:1108–1120
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14:557–577
Cervený J, Šetlík I, Trtílek M, Nedbal L (2009) Photobioreactor for cultivation and real-time, in-situ measurement of O2 and CO2 exchange rates, growth dynamics, and of chlorophyll fluorescence emission of photoautotrophic microorganisms. Eng Life Sci 9:1–7
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Darzins A, Pienkos P, Edye L (2010) Current status and potential for algal biofuels production. Report prepared for the International Energy Agency, Bioenergy Task 39, Report T39-T2. 6 August 2010, National Renewable Energy Laboratory and BioIndustry Partners, Golden, Colorado, p 131. www.task39.org
Eriksen N (2008) The technology of microalgal culturing. Biotechnol Lett 30:1525–1536
Fabregas J, Abalde J, Herrero C, Cabezas B, Veiga M (1984) Growth of the marine microalga Tetraselmis suecica in batch cultures with different salinities and nutrient concentrations. Aquaculture 42:207–215
Fernández F, Pérez J, Sevilla J, Camacho F, Grima E (2000) Modeling of eicosapentaenoic acid (EPA) production from Phaeodactylum tricornutum cultures in tubular photobioreactors. Effects of dilution rate, tube diameter, and solar irradiance. Biotech Bioeng 68:173–183
Fon Sing M (2010) Strain selection and outdoor cultivation of halophilic microalgae with potential for large-scale biodiesel production. PhD thesis. Murdoch University, Perth 212 p
Fon Sing S, Isdepsky A, Borowitzka M, Moheimani N (2013) Production of biofuels from microalgae. Mitig Adapt Strateg Glob Chang 18:47–72
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Grobbelaar JU (2009) Factors governing algal growth in photobioreactors: the “open” versus “closed” debate. J Appl Phycol 21:489–492
Grobbelaar JU (2012) Microalgae mass culture: the constraints of scaling-up. J Appl Phycol 24:315–318
Guillard RRL (1973) Division rates. In: Stein JR (ed) Handbook of phycological methods: culture methods and growth measurements. Cambridge University Press, Cambridge, pp 289–311
Guillard R, Ryther J (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Hayes A (1978) A pH stat for carbon dioxide incubator control. J Clin Pathol 31:696–699
Hori T, Norris R, Chihara M (1982) Studies on the ultrastructure and taxonomy of the genus Tetraselmis (Prasinophyceae). J Plant Res 95:49–61
Isdepsky A, Raes EJ, Moheimani NR, Borowitzka MA (2010) Impact of hydrodynamics and light on cell growth in open raceway ponds and an enclosed Biocoil. In: Poster presented at the Biomass for a Clean Energy Future conference, Sydney, Australia, 2010
Janssen M, Tramper J, Mur LR, Wijffels RH (2003) Enclosed outdoor photobioreactors: light regime, photosynthetic efficiency, scale-up, and future prospects. Biotech Bioeng 81:193–210
Kirst GO (1979) Osmotische Adaption der marinen Planktonalge Platymonas subcordiformis. Ber Deut Bot Ges 92:31–42
Leupold M, Hindersin S, Gust G, Kerner M, Hanelt D (2013) Influence of mixing and shear stress on Chlorella vulgaris, Scenedesmus obliquus, and Chlamydomonas reinhardtii. J Appl Phycol 25:485–495
Mercz T (1994) A study of high lipid yielding microalgae with potential for large-scale production of lipids and polyunsaturated fatty acids. PhD thesis, Murdoch University, Perth
Moheimani N, Borowitzka M (2006) Limits to productivity of the alga Pleurochrysis carterae (Haptophyta) grown in outdoor raceway ponds. Biotech Bioeng 96:27–36
Moheimani NR, Isdepsky A, Lisec J, Raes E, Borowitzka MA (2011) Coccolithophorid algae culture in closed photobioreactors. Biotech Bioeng 108:2078–2087
Moheimani N, Borowitzka M, Isdepsky A, Fon Sing S (2013) Standard methods for measuring growth of algae and their composition. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer, Dordrecht, pp 265–284
Molina E, Fernández J, Acién FG, Chisti Y (2001) Tubular photobioreactor design for algal cultures. J Biotechnol 92:113–131
Norris R, Hori T, Chihara M (1980) Revision of the genusTetraselmis (Class Prasinophyceae). J Plant Res 93:317–339
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648
Richmond A, Hu Q (eds) (2013) Handbook of microalgal culture: applied phycology and biotechnology. Wiley-Blackwell, Chichester, p 719
Richmond A, Zhang C, Zarmi Y (2003) Efficient use of strong light for high photosynthetic productivity: interrelationships between the optical path, the optimal population density and cell-growth inhibition. Biomol Eng 20:229–236
Robinson L, Morrison A, Bamforth M (1988) Improvements relating to biosynthesis. European Patent 261, 872
Tredici MR, Zittelli GC (1998) Efficiency of sunlight utilization: tubular versus flat photobioreactors. Biotech Bioeng 57:187–197
Ugwu C, Ogbonna J, Tanaka H (2005) Light/dark cyclic movement of algal culture (Synechocystis aquatilis) in outdoor inclined tubular photobioreactor equipped with static mixers for efficient production of biomass. Biotechnol Lett 27:75–78
van Beilen J (2010) Why microalgal biofuels won't save the internal combustion machine. Biofuels Bioprod Bioref 4:41–52
Zitelli GC, Rodolfi L, Bassi N, Biondi N, Tredici MR (2013) Photobioreactors for biofuel production. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer, Dordrecht, pp 115–131
Acknowledgments
This project received partial funding from the Australian Government as part of the Asia-Pacific Partnership (APP) on Clean Development and Climate. The views expressed herein are not necessarily the views of the Commonwealth, and the Commonwealth does not accept responsibility for any information or advice contained herein.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raes, E.J., Isdepsky, A., Muylaert, K. et al. Comparison of growth of Tetraselmis in a tubular photobioreactor (Biocoil) and a raceway pond. J Appl Phycol 26, 247–255 (2014). https://doi.org/10.1007/s10811-013-0077-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-0077-5