Abstract
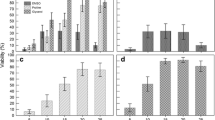
This study investigated the toxicity and effectiveness of various cryoprotectant solutions to develop a cryopreservation technique by one-step fast cooling for gametophytic thalli of Porphyra yezoensis. Dimethyl sulfoxide (DMSO) was not harmful at concentrations up to 30 % after 30 s of immersion. However, an increase in concentration beyond 40 % was seriously detrimental, and the detrimental effect occurred at lower concentrations in the case of 60 s of immersion. Diglycerol was the least harmful among cryoprotectants tested, and its harmful effect was not found at concentrations up to 50 %, even after 60 s of immersion. The thalli loaded into a straw were directly plunged into liquid nitrogen after 30 s of immersion in various cryoprotectant solutions. When 5 % DMSO was added to diglycerol, high survival rates (more than 80 %) were achieved at diglycerol concentrations of 15–25 %, and the maximum survival was 94.6 % at diglycerol concentrations of 20 %. The thawed thalli restarted growth without any difference in comparison with unfrozen thalli.


Similar content being viewed by others
References
Chen THH, Kartha KK, Leung NL, Kurz WGW, Chatson KB, Constabel F (1984) Cryopreservation of alkaloid-producing cell cultures of periwinkle (Catharanthus roseus). Plant Physiol 75:726–731
Day JG, Watanabe MM, Morris GJ, Fleck RA, McLellan MR (1997) Long-term viability of preserved eukaryotic algae. J Appl Phycol 9:121–127
Edashige K, Valdez DM Jr, Hara T, Saida N, Seki S, Kasai M (2006) Japanese flounder (Paralichthys olivaceus) embryos are difficult to cryopreserve by vitrification. Cryobiology 53:96–106
Fahy GM, MacFarlane DR, Angell CA, Meryman HT (1984) Vitrification as an approach to cryopreservation. Cryobiology 21:407–426
Hirai D, Sakai A (1999) Cryopreservation of in vitro-grown meristems of potato (Solanum tuberosum L.) by encapsulation-vitrification. Potato Res 42:153–160
Hirata R, Takahashi M, Saga N, Mikami K (2011) Transient gene expression system established in Porphyra yezoensis is widely applicable in Bangiophycean algae. Mar Biotechnol 13:1038–1047
Kono S, Kuwano K, Ninomiya M, Onishi J, Saga N (1997) Cryopreservation of Enteromorpha intestinalis (Ulvales, Chlorophyta) in liquid nitrogen. Phycologia 36:76–78
Kono S, Kuwano K, Saga N (1998) Cryopreservation of Eisenia bicyclis (Laminariales, Phaeophyta) in liquid nitrogen. J Mar Biotech 6:220–223
Kuwano K, Saga N (2000) Cryopreservation of marine algae: applications in biotechnology. In: Fingerman M, Nagabhushaman R (eds) Recent advances in marine biotechnology, Volume 4: Aquaculture. Science Publisher Inc, New Hampshire, pp 23–40
Kuwano K, Aruga Y, Saga N (1993) Cryopreservation of the conchocelis of the marine alga Porphyra yezoensis Ueda (Rhodophyta) in liquid nitrogen. Plant Sci 94:215–225
Kuwano K, Aruga Y, Saga N (1996) Cryopreservation of clonal gametophytic thalli of Porphyra (Rhodophyta). Plant Sci 116:117–124
Kuwano K, Matsuka S, Kono S, Ninomiya M, Onishi J, Saga N (1998) Growth and the content of laurinterol and debromolaurinterol in Laurencia okamurae (Ceramiales, Rhodophyta). J Appl Phycol 10:9–14
Liu H, Yu W, Dai J, Gong Q, Yang K, Lu X (2004) Cryopreservation of protoplasts of the alga Porphyra yezoensis by vitrification. Plant Sci 166:97–102
Martínez-Burgos M, Herrero L, Megías D, Salvanes R, Montoya MC, Cobo AC, Garcia-Velasco JA (2011) Vitrification versus slow freezing of oocytes: effects on morphologic appearance, meiotic spindle configuration, and DNA damage. Fertil Steril 95:374–377
Mazur P (1984) Freezing of living cells: mechanisms and implications. Am J Physiol 247:C125–C142
Mumford TF Jr, Miura A (1988) Porphyra as food: cultivation and economics. In: Lembi CA, Waaland JR (eds) Algae and human affairs. Cambridge University Press, Cambridge, pp 87–117
Nakagata N (1989) High survival rate of unfertilized mouse oocytes after vitrification. J Reprod Fert 87:479–483
Nakanishi K, Deuchi K, Kuwano K (2012) Cryopreservation of four valuable strains of microalgae, including viability and characteristics during 15 years of cryostorage. J Appl Phycol. doi:10.1007/s10811-012-9790-8
Park J-I, Lee J, Sim SJ, Lee J-H (2009) Production of hydrogen from marine macro-algae biomass using anaerobic sewage sludge microflora. Biotechnol Bioprocess Eng 14:307–315
Provasoli L (1968) Media and prospects for the cultivation of marine algae. In: Watanabe A, Hattori A (eds) Cultures and collections of algae, Proc. US–Japan Conf, Hakone, September 1966, Jpn Soc Plant Physiol, Kyoto, pp. 63–75
Rall WF, Fahy GM (1985) Ice-free cryopreservation of mouse embryos at −196°C by vitrification. Nature 313:573–575
Saga N, Sakanishi Y, Ogishima T (1989) Method for quick evaluation of cell viability in marine macroalgae. Jpn J Phycol 37:129–136
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Steponkus PL (1984) Role of the plasma membrane in freezing injury and cold acclimation. Annu Rev Plant Physiol 35:543–584
Takagi H, Thinh NT, Islam OM, Senboku T, Sakai A (1997) Cryopreservation of in vitro-grown shoot tips of taro (Colocasia esculenta (L.) Schott) by vitrification. 1. Investigation of basic conditions of the vitrification procedure. Plant Cell Reports 16:594–599
Taylor R, Fletcher RL (1998) Cryopreservation of eukaryotic algae—a review of methodologies. J Appl Phycol 10:481–501
Uragami A, Sakai A, Nagai M, Takahashi T (1989) Survival of cultured cells and somatic embryos of Asparagus officinalis cryopreserved by vitrification. Plant Cell Rep 8:418–421
Velasquez-Orta SB, Curtis TP, Logan BE (2009) Energy from algae using microbial fuel cells. Biotechnol Bioeng 103:1068–1076
Acknowledgments
This study was supported in part by a grant-in-aid for scientific research (no. 22580379) to K.K. from the Ministry of Education, Science and Culture, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, Y.H., Nam, T.J. & Kuwano, K. Cryopreservation of gametophytic thalli of Porphyra yezoensis (Rhodophyceae) by one-step fast cooling. J Appl Phycol 25, 531–535 (2013). https://doi.org/10.1007/s10811-012-9887-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9887-0