Abstract
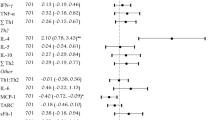
We examined associations between prenatal oxidative stress (OS) and child autism-related outcomes. Women with an autistic child were followed through a subsequent pregnancy and that younger sibling’s childhood. Associations between glutathione (GSH), glutathione disulfide (GSSG), 8-oxo-deoxyguanine (8-OHdG), and nitrotyrosine and younger sibling Social Responsiveness Scale (SRS) scores were examined using quantile regression. Increasing GSH:GSSG (suggesting decreasing OS) was associated with minor increases in SRS scores (50th percentile β: 1.78, 95% CI: 0.67, 3.06); no other associations were observed. Results from this cohort with increased risk for autism do not support a strong relationship between OS in late pregnancy and autism-related outcomes. Results may be specific to those with enriched autism risk; future work should consider other timepoints and biomarkers.


Similar content being viewed by others
References
Atamer, Y., Koçyigit, Y., Yokus, B., Atamer, A., & Erden, A. C. (2005). Lipid peroxidation, antioxidant defense, status of trace metals and leptin levels in preeclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology, 119(1), 60–66
Berr, C., Balansard, B., Arnaud, J., Roussel, A. M., Alpérovitch, A., & Group, E. S. (2000). Cognitive decline is associated with systemic oxidative stress: the EVA study. Journal of the American Geriatrics Society, 48(10), 1285–1291
Birben, E., Sahiner, U. M., Sackesen, C., Erzurum, S., & Kalayci, O. (2012). Oxidative stress and antioxidant defense. World Allergy Organization Journal, 5(1), 9–19
Bishop, S. L., Guthrie, W., Coffing, M., & Lord, C. (2011). Convergent validity of the Mullen Scales of Early Learning and the differential ability scales in children with autism spectrum disorders. American journal on intellectual and developmental disabilities, 116(5), 331–343
Boyle, C. A., Boulet, S., Schieve, L. A., Cohen, R. A., Blumberg, S. J., Yeargin-Allsopp, M., & Kogan, M. D. (2011). Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics, 127(6), 1034–1042
Constantino, J. N., Davis, S. A., Todd, R. D., Schindler, M. K., Gross, M. M., Brophy, S. L., & Reich, W. (2003). Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of autism and developmental disorders, 33(4), 427–433
Constantino, J. N., & Gruber, C. P. (2012). The social responsiveness scale (2nd ed.).). Western Psychological Services
Dennery, P. A. (2010). Oxidative stress in development: nature or nurture? Free Radical Biology and Medicine, 49(7), 1147–1151
DeVilbiss, E. A., Gardner, R. M., Newschaffer, C. J., & Lee, B. K. (2015). Maternal folate status as a risk factor for autism spectrum disorders: a review of existing evidence. British Journal of Nutrition, 114(5), 663–672
Goodrich, A. J., Volk, H. E., Tancredi, D. J., McConnell, R., Lurmann, F. W., Hansen, R. L., & Schmidt, R. J. (2018). Joint effects of prenatal air pollutant exposure and maternal folic acid supplementation on risk of autism spectrum disorder. Autism Research, 11(1), 69–80
Hajjar, I., Hayek, S. S., Goldstein, F. C., Martin, G., Jones, D. P., & Quyyumi, A. (2018). Oxidative stress predicts cognitive decline with aging in healthy adults: an observational study. Journal of Neuroinflammation, 15(1), 1–7
Hallmayer, J., Cleveland, S., Torres, A., Phillips, J., Cohen, B., Torigoe, T., & Smith, K. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Archives of general psychiatry, 68(11), 1095–1102
Hollowood, K., Melnyk, S., Pavliv, O., Evans, T., Sides, A., Schmidt, R. J., & Kruger, U. (2018). Maternal metabolic profile predicts high or low risk of an autism pregnancy outcome. Research in autism spectrum disorders, 56, 72–82
Howsmon, D. P., Kruger, U., Melnyk, S., James, S. J., & Hahn, J. (2017). Classification and adaptive behavior prediction of children with autism spectrum disorder based upon multivariate data analysis of markers of oxidative stress and DNA methylation.PLoS computational biology, 13(3), e1005385
James, S. J., Cutler, P., Melnyk, S., Jernigan, S., Janak, L., Gaylor, D. W., & Neubrander, J. A. (2004). Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. The American journal of clinical nutrition, 80(6), 1611–1617
James, S. J., Melnyk, S., Jernigan, S., Cleves, M. A., Halsted, C. H., Wong, D. H., & Bradstreet, J. J. (2006). Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 141(8), 947–956
Jenabi, E., Karami, M., Khazaei, S., & Bashirian, S. (2019). The association between preeclampsia and autism spectrum disorders among children: a meta-analysis. Korean journal of pediatrics, 62(4), 126–130. doi:https://doi.org/10.3345/kjp.2018.07010
Jones, D. P. (2002). [11] Redox potential of GSH/GSSG couple: assay and biological significance. Methods in enzymology, 348, 93–112
Kim, G. H., Kim, J. E., Rhie, S. J., & Yoon, S. (2015). The role of oxidative stress in neurodegenerative diseases. Experimental neurobiology, 24(4), 325
quantreg: Quantile Regression. R package version 5.85. (2021). Koenker, R. [Mobile application software]
Koenker, R., & Hallock, K. F. (2001). Quantile regression. Journal of economic perspectives, 15(4), 143–156
Lanté, F., Meunier, J., Guiramand, J., Maurice, T., Cavalier, M., de Jesus Ferreira, M. C., & Barbanel, G. (2007). Neurodevelopmental damage after prenatal infection: Role of oxidative stress in the fetal brain. Free Radical Biology and Medicine, 42(8), 1231–1245. doi:https://doi.org/10.1016/j.freeradbiomed.2007.01.027
Lawson, J. A., FitzGerald, G. A., & Rokach, J. (1999). Isoprostanes: formation, analysis and use as indices of lipid peroxidation in vivo. Journal of Biological Chemistry, 274(35), 24441–24444
London, E. B. (2014). Categorical diagnosis: a fatal flaw for autism research? Trends in neurosciences, 37(12), 683–686
López-Tinoco, C., Roca, M., García-Valero, A., Murri, M., Tinahones, F. J., Segundo, C., & Aguilar-Diosdado, M. (2013). Oxidative stress and antioxidant status in patients with late-onset gestational diabetes mellitus. Acta Diabetologica, 50(2), 201–208. doi:https://doi.org/10.1007/s00592-011-0264-2
Lord, C., Rutter, M., Goode, S., Heemsbergen, J., Jordan, H., Mawhood, L., & Schopler, E. (1989). Austism diagnostic observation schedule: A standardized observation of communicative and social behavior. Journal of autism and developmental disorders, 19(2), 185–212
Lyall, K., Croen, L., Daniels, J., Fallin, M. D., Ladd-Acosta, C., Lee, B. K., & Volk, H. (2017). The changing epidemiology of autism spectrum disorders. Annual review of public health, 38, 81–102
Lyall, K., Pauls, D. L., Spiegelman, D., Ascherio, A., & Santangelo, S. L. (2012). Pregnancy complications and obstetric suboptimality in association with autism spectrum disorders in children of the Nurses’ Health Study II. Autism Research, 5(1), 21–30
Maenner, M. J., Shaw, K. A., Baio, J., EdS, Washington, A., Patrick, M., Dietz, P. M., & United States. (2020). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, 2016. Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C.: 2002), 69(4), 1–12. doi:https://doi.org/10.15585/mmwr.ss6904a1
Matsubasa, T., Uchino, T., Karashima, S., Kondo, Y., Maruyama, K., Tanimura, M., & Endo, F. (2002). Oxidative stress in very low birth weight infants as measured by urinary 8-OHdG. Free Radical Biology and Medicine, 36(2), 189–193
Melnyk, S., Fuchs, G. J., Schulz, E., Lopez, M., Kahler, S. G., Fussell, J. J., & Seidel, L. (2012). Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism. Journal of autism and developmental disorders, 42(3), 367–377
Moore, T. A., Ahmad, I. M., Schmid, K. K., Berger, A. M., Ruiz, R. J., Pickler, R. H., & Zimmerman, M. C. (2019). Oxidative stress levels throughout pregnancy, at birth, and in the neonate. Biological research for nursing, 21(5), 485–494
Mullen, E. M. (1995). Mullen scales of early learning manual. American Guidance Service
Neggers, Y. (2014). The Relationship between Folic Acid and Risk of Autism Spectrum Disorders. Healthcare (Basel Switzerland), 2(4), 429–444. doi:https://doi.org/10.3390/healthcare2040429
Newschaffer, C. J., Croen, L. A., Fallin, M. D., Hertz-Picciotto, I., Nguyen, D. V., Lee, N. L., & Shedd-Wise, K. M. (2012). Infant siblings and the investigation of autism risk factors. Journal of Neurodevelopmental Disorders, 4(1), 7. doi:https://doi.org/10.1186/1866-1955-4-7
Ozonoff, S., Young, G. S., Belding, A., Hill, M., Hill, A., Hutman, T., & Schwichtenberg, A. (2014). The broader autism phenotype in infancy: when does it emerge? Journal of the American Academy of Child & Adolescent Psychiatry, 53(4), 398–407. e392
Olsson, M. G., Centlow, M., Rutardóttir, S., Stenfors, I., Larsson, J., Hosseini-Maaf, B. … Akerström, B. (2010). Increased levels of cell-free hemoglobin, oxidation markers, and the antioxidative heme scavenger alpha(1)-microglobulin in preeclampsia. Free Radical Biology and Medicine, 48(2), 284–291
Patti, M. A., Newschaffer, C., Eliot, M., Hamra, G. B., Chen, A., Croen, L. A., & Khoury, J. C. (2021). Gestational exposure to phthalates and social responsiveness scores in children using quantile regression: The EARLI and HOME studies. International journal of environmental research and public health, 18(3), 1254
Postorino, V., Fatta, L., Sanges, V., Giovagnoli, G., De Peppo, L., Vicari, S., & Mazzone, L. (2016). Intellectual disability in autism spectrum disorder: investigation of prevalence in an Italian sample of children and adolescents
Rice, D., & Barone, S. Jr. (2000). Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental health perspectives, 108(suppl 3), 511–533
Risch, N., Hoffmann, T. J., Anderson, M., Croen, L. A., Grether, J. K., & Windham, G. C. (2014). Familial recurrence of autism spectrum disorder: evaluating genetic and environmental contributions. American Journal of Psychiatry, 171(11), 1206–1213
Rommel, A. S., Milne, G. L., Barrett, E. S., Bush, N. R., Nguyen, R., Sathyanarayana, S., & Ferguson, K. K. (2020). Associations between urinary biomarkers of oxidative stress in the third trimester of pregnancy and behavioral outcomes in the child at 4 years of age. Brain Behavior and Immunity, 90, 272–278
Rose, S., Melnyk, S., Pavliv, O., Bai, S., Nick, T., Frye, R., & James, S. (2012). Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Translational psychiatry, 2(7), e134–e134
Sagiv, S. K., Kalkbrenner, A. E., & Bellinger, D. C. (2015). Of decrements and disorders: assessing impairments in neurodevelopment in prospective studies of environmental toxicant exposures. Environmental Health, 14(1), 1–4
Sbodio, J. I., Snyder, S. H., & Paul, B. D. (2019). Regulators of the transsulfuration pathway. British journal of pharmacology, 176(4), 583–593. doi:https://doi.org/10.1111/bph.14446
Schmidt, R. J., Hansen, R. L., Hartiala, J., Allayee, H., Schmidt, L. C., Tancredi, D. J., & Hertz-Picciotto, I. (2011). Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology (Cambridge Mass), 22(4), 476–485. doi:https://doi.org/10.1097/EDE.0b013e31821d0e30
Shelton, J. F., Hertz-Picciotto, I., & Pessah, I. N. (2012). Tipping the balance of autism risk: potential mechanisms linking pesticides and autism. Environmental health perspectives, 120(7), 944–951
Svatikova, A., Wolk, R., Wang, H. H., Otto, M. E., Bybee, K. A., Singh, R. J., & Somers, V. K. (2004). Circulating free nitrotyrosine in obstructive sleep apnea. American Journal of Physiology-Regulatory Integrative and Comparative Physiology, 287(2), R284–R287
Thompson, L. P., & Al-Hasan, Y. (2012). Impact of oxidative stress in fetal programming. Journal of pregnancy, 2012
Toboła-Wróbel, K., Pietryga, M., Dydowicz, P., Napierała, M., Brązert, J., & Florek, E. (2020). Association of Oxidative Stress on Pregnancy. Oxidative Medicine and Cellular Longevity, 2020, 6398520. doi:https://doi.org/10.1155/2020/6398520
Valavanidis, A., Vlachogianni, T., & Fiotakis, C. (2009). 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. Journal of environmental science and health Part C, 27(2), 120–139
Van Naarden Braun, K., Christensen, D., Doernberg, N., Schieve, L., Rice, C., Wiggins, L., & Yeargin-Allsopp, M. (2015). Trends in the prevalence of autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan Atlanta, 1991–2010. PloS one, 10(4), e0124120
Wells, P. G., Bhatia, S., Drake, D. M., & Miller-Pinsler, L. (2016). Fetal oxidative stress mechanisms of neurodevelopmental deficits and exacerbation by ethanol and methamphetamine. Birth Defects Research Part C: Embryo Today: Reviews, 108(2), 108–130
Wells, P. G., McCallum, G. P., Chen, C. S., Henderson, J. T., Lee, C. J., Perstin, J., & Wong, A. W. (2009). Oxidative stress in developmental origins of disease: teratogenesis, neurodevelopmental deficits, and cancer. Toxicological sciences, 108(1), 4–18
Acknowledgements
The authors would like to thank Drs. Marisa Patti and Loni Tabb for their guidance in statistical modeling.
Funding
This research was supported by the Eunice Shriver Kennedy National Institute of Child Health and Human Development (NICHD) at the National Institutes of Health (NIH) (R21HD096356). The EARLI Study was funded by the National Institute of Environmental Health Sciences, the National Institute of Mental Health, the National Institute of Child Health and Human Development, and the National Institute of Neurologic Disease and Stroke (R01 ES016443; R24 ES030893), with additional funding from Autism Speaks (AS 5938).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Lisa A. Croen, M. Daniele Fallin, Irva Hertz-Picciotto, and Craig Newschaffer. Biomarker analysis was conducted by S. Jill James, Stepan Melnyk, and Nathaniel Snyder. Statistical analyses were completed by Meghan Carey and Juliette Rando. The first draft of the manuscript was written by Meghan Carey and Kristen Lyall and all authors edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was approved by the Drexel University Institutional Review Board (IRB). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carey, M.E., Rando, J., Melnyk, S. et al. Examining associations between prenatal biomarkers of oxidative stress and ASD-related outcomes using quantile regression. J Autism Dev Disord 53, 2975–2985 (2023). https://doi.org/10.1007/s10803-022-05625-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-022-05625-9