Abstract
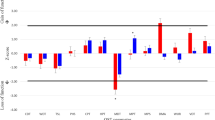
We tested endogenous pain modulation mechanisms in adults with autism spectrum disorders (ASD). Nineteen ASD adults without intellectual disabilities were included, matched with 19 healthy volunteers on the basis of sex and chronological age. An experimental pain model was used to measure excitatory and inhibitory pain mechanisms in a single session. Statistical analyses indicated that endogenous pain modulation mechanisms in ASD group did not differ significantly from those of healthy adults. The pain scores were very disparate in ASD group with a greater range of extreme scores than in control group. Unlike schizophrenic patients, there was no systematic dysfunction of endogenous excitatory pain modulation mechanisms, but the high variability requires to be wise to interpret the results and formulate conclusion

Similar content being viewed by others
References
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). Philadelphia: American Psychiatric Publishing.
Arendt-Nielsen, L., Brennum, J., Sindrup, S., & Bak, P. (1994). Electrophysiological and psychophysical quantification of temporal summation in the human nociceptive system. European Journal of Applied Physiology and Occupational Physiology,68(3), 266–273.
Autié, A., Montreuil, M., Moulier, V., Braha, S., Wojakiewicz, A., & Januel, D. (2009). Pain and schizophrenia: Myth and reality. Encephale,35(4), 297–303.
Barrett, K. E., Barman, S. M., Boitano, S., & Brooks, H. (2009). Ganong’s review of medical physiology. New York: McGraw-Hill Medical.
Baum, S. H., Stevenson, R. A., & Wallace, M. T. (2015). Behavioral, perceptual, and neural alterations in sensory and multisensory function in autism spectrum disorder. Progress in Neurobiology,134, 140–160.
Beck, A. T., Steer, R. A., & Brown, G. K. (1998). Inventaire de Dépression de Beck. Manuel (2nd ed.). Toronto: Psychological Corporation.
Bonnot, O., Anderson, G. M., Cohen, D., Willer, J. C., & Tordjman, S. (2009). Are patients with schizophrenia insensitive to pain? A reconsideration of the question. Clinical Journal of Pain,25(3), 244–252.
Brown, C., & Dunn, W. (2002). The adult sensory profile. San Antonio, TX: The Psychological Corporation.
Cascio, C., McGlone, F., Folger, S., et al. (2008). Tactile perception in adults with autism: A multidimensional psychophysical study. Journal of Autism and Developmental Disorders,38, 127–137.
Chen, C., Hung, A. Y., Fan, Y. T., Tan, S., Hong, H., & Cheng, Y. (2017). Linkage between pain sensitivity and empathic response in adolescents with autism spectrum conditions and conduct disorder symptoms. Autism Research,10(2), 267–275.
Craig, K. D. (2009). The social communication model of pain. Canadian Psychology,50(1), 22.
Dickens, C., McGowan, L., & Dale, S. (2003). Impact of depression on experimental pain perception: A systematic review of the literature with meta-analysis. Psychosomatic Medicine,65(3), 369–375.
Dubois, A., Michelon, C., Rattaz, C., Zabalia, M., & Baghdadli, A. (2017). Daily living pain assessment in children with autism: Exploratory study. Research in Developmental Disabilities,62, 238–246.
Dubois, A., Rattaz, C., Pry, R., & Baghdadli, A. (2010). Autism and pain: A literature review. Pain Research & Management,15(4), 245–253.
Duerden, E. G., Taylor, M. J., Lee, M., McGrath, P. A., Davis, K. D., & Roberts, S. W. (2015). Decreased sensitivity to thermal stimuli in adolescents with autism spectrum disorder: Relation to symptomatology and cognitive ability. The Journal of Pain,16(5), 463–471.
Failla, M., Davis, S., Gerdes, M., Williams, Z., Moore, D., & Cascio, C. (2019). (262) Increased heat pain sensitivity and pain-related anxiety in individuals with autism. The Journal of Pain,20(4), S40.
Fründt, O., Grashorn, W., Schöttle, D., Peiker, I., David, N., Engel, A. K., … & Bingel, U. (2017). Quantitative sensory testing in adults with autism spectrum disorders. Journal of Autism and Developmental Disorders, 47(4), 1183–1192.
Furukawa, T. A. (2010). Assessment of mood: Guides for clinicians. Journal of Psychosomatic Research,68(6), 581–589.
Gilbert-MacLeod, C. A., Craig, K. D., Rocha, E. M., & Mathias, M. D. (2000). Everyday pain responses in children with and without developmental delays. Journal of Pediatric Psychology,25(5), 301–308.
Gillberg, C., & Coleman, M. (1996). Autism and medical disorders: A review of the literature. Developmental Medicine and Child Neurology,38(3), 191–202.
Goffaux, P., Redmond, W. J., Rainville, P., & Marchand, S. (2007). Descending analgesia-when the spine echoes what the brain expects. Pain,130(1–2), 137–143.
Granot, M., Granovsky, Y., Sprecher, E., Nir, R. R., & Yarnitsky, D. (2006). Contact heat-evoked temporal summation: Tonic versus repetitive-phasic stimulation. Pain,122(3), 295–305.
Granot, M., Sprecher, E., & Yarnitsky, D. (2003). Psychophysics of phasic and tonic heat pain stimuli by quantitative sensory testing in healthy subjects. European Journal of Pain,7(2), 139–143.
Hadjistavropoulos, T., & Craig, K. D. (2002). A theoretical framework for understanding self-report and observational measures of pain: A communications model. Behaviour Research and Therapy,40(5), 551–570.
Joshi, G., Wozniak, J., Petty, C., Martelon, M. K., Fried, R., Bolfek, A., … & Caruso, J. (2013). Psychiatric comorbidity and functioning in a clinically referred population of adults with autism spectrum disorders: A comparative study. Journal of Autism and Developmental Disorders, 43(6), 1314–1325.
Julien, N., Goffaux, P., Arsenault, P., & Marchand, S. (2005). Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain,114(1), 295–302.
Kennedy, D. L., Kemp, H. I., Ridout, D., Yarnitsky, D., & Rice, A. S. (2016). Reliability of conditioned pain modulation: A systematic review. Pain,157(11), 2410.
Le Bars, D., Dickenson, A. H., & Besson, J. M. (1979). Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain,6(3), 283–304.
Le Bars, D., Villanueva, L., Bouhassira, D., & Willer, J. C. (1992). Diffuse noxious inhibitory controls (DNIC) in animals and in man. Patologicheskaia fiziologiia i eksperimental’naia terapiia,4, 55–65.
Lee, L. C., Harrington, R. A., Chang, J. J., & Connors, S. L. (2008). Increased risk of injury in children with developmental disabilities. Research in Developmental Disabilities,29(3), 247–255.
Lévesque, M., Potvin, S., Marchand, S., Stip, E., Grignon, S., Pierre, L., … & Goffaux, P. (2012). Pain perception in schizophrenia: Evidence of a specific pain response profile. Pain Medicine, 13(12), 1571–1579.
Lord, C., Rutter, M., & Le Couteur, A. (1994). Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders,24(5), 659–685.
Magerl, W., & Klein, T. (2006). Experimental human models of neuropathic pain. Handbook of clinical neurology (Vol. 81, pp. 503–516). New York: Elsevier.
Marchand, S., & Arsenault, P. (2002). Spatial summation for pain perception: Interaction of inhibitory and excitatory mechanisms. Pain,95(3), 201–206.
Messmer, R. L., Nader, R., & Craig, K. D. (2008). Brief report: Judging pain intensity in children with autism undergoing venepuncture: The influence of facial activity. Journal of Autism and Developmental Disorders,38(7), 1391–1394.
Militerni, R., Bravaccio, C., Falco, C., Puglisi-Allegra, S., Pascucci, T., & Fico, C. (2000). Pain reactivity in children with autistic disorder. The Journal of Headache and Pain,1(1), 53–56.
Moore, D. J. (2015). Acute pain experience in individuals with autism spectrum disorders: A review. Autism,19(4), 387–399.
Morin, M., Marchand, S., Couturier, L., Nadeau, S., & Lafrenaye, S. (2014). Long-term persistency of abnormal heart rate variability following long NICU stay and surgery at birth. Pain Research and Treatment,2014, 121289.
Nader, R., Oberlander, T. F., Chambers, C. T., & Craig, K. D. (2004). Expression of pain in children with autism. The Clinical Journal of Pain,20(2), 88–97.
Potvin, S., Paul-Savoie, E., Morin, M., Bourgault, P., & Marchand, S. (2012). Temporal summation of pain is not amplified in a large proportion of fibromyalgia patients. Pain Research and Treatment,2012, 938595.
Potvin, S., Stip, E., Tempier, A., Pampoulova, T., Bentaleb, L. A., Lalonde, P., et al. (2008). Pain perception in schizophrenia: No changes in diffuse noxious inhibitory controls (DNIC) nut a lack of pain sensitization. Journal of Psychiatric Research,42(12), 1010–1016.
Pud, D., Granovsky, Y., & Yarnitsky, D. (2009). The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain,144(1), 16–19.
Rainville, P., Feine, J. S., Bushnell, M. C., & Duncan, G. H. (1992). A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. Somatosensory and Motor Research,9(4), 265–277.
Rattaz, C., Dubois, A., & Baghdadli, A. (2016). How do people with autism spectrum disorders (ASD) experience pain? An introduction to pain and its relation to nervous system disorders (pp. 295–315). New York: Wiley.
Rattaz, C., Dubois, A., Michelon, C., Viellard, M., Poinso, F., & Baghdadli, A. (2013). How do children with autism spectrum disorders express pain? A comparison with developmentally delayed and typically developing children. Pain,154(10), 2007–2013.
Riquelme, I., Hatem, S. M., & Montoya, P. (2016). Abnormal pressure pain, touch sensitivity, proprioception, and manual dexterity in children with autism spectrum disorders. Neural Plasticity. https://doi.org/10.1155/2016/1723401.
Serrao, M., Rossi, P., Sandrini, G., Parisi, L., Amabile, G. A., Nappi, G., et al. (2004). Effects of diffuse noxious inhibitory controls on temporal summation of the RIII reflex in humans. Pain,112(3), 353–360.
Staud, R., Robinson, M. E., Vierck, C. J., Jr., & Price, D. D. (2003). Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain,101(1–2), 167–174.
Stubbs, B., Thompson, T., Acaster, S., Vancampfort, D., Gaughran, F., & Correll, C. U. (2015). Decreased pain sensitivity among people with schizophrenia: A meta-analysis of experimental pain induction studies. Pain,156(11), 2121–2131.
Sullivan, M. J., Thorn, B., Haythornthwaite, J. A., Keefe, F., Martin, M., Bradley, L. A., et al. (2001). Theoretical perspectives on the relation between catastrophizing and pain. The Clinical Journal of Pain,17(1), 52–64.
Thompson, T., Correll, C. U., Gallop, K., Vancampfort, D., & Stubbs, B. (2016). Is pain perception altered in people with depression? A systematic review and meta-analysis of experimental pain research. The Journal of Pain,17(12), 1257–1272.
Tordjman, S., Anderson, G. M., Botbol, M., Brailly-Tabard, S., Perez-Diaz, F., Graignic, R., … & Trabado, S. (2009). Pain reactivity and plasma β-endorphin in children and adolescents with autistic disorder. PLoS ONE, 4(8), e5289.
Tordjman, S., Antoine, C., Cohen, D. J., Gauvain-Piquard, A., Carlier, M., Roubertoux, P., et al. (1999). Study of the relationships between self-injurious behavior and pain reactivity in infantile autism. L’encéphale,25(2), 122–134.
Tousignant-Laflamme, Y., Pagé, S., Goffaux, P., & Marchand, S. (2008). An experimental model to measure excitatory and inhibitory pain mechanisms in humans. Brain Research,1230, 73–79.
Vaughan, S., McGlone, F., Poole, H., & Moore, D. J. (2019). A quantitative sensory testing approach to pain in autism spectrum disorders. Journal of Autism and Developmental Disorders,4, e5289.
Wechsler, D. (2008). Wechsler adult intelligence scale (WAIS-IV) (4th ed., Vol. 22, p. 498). San Antonio, TX: NCS Pearson.
Willer, J. C., De Broucker, T., & Le Bars, D. (1989). Encoding of nociceptive thermal stimuli by diffuse noxious inhibitory controls in humans. Journal of Neurophysiology,62(5), 1028–1038.
World Health Organization. (1993). The ICD-10 classification of mental and behavioural disorders: Diagnostic criteria for research (Vol. 2). Geneva: World Health Organization.
Yarnitsky, D. (2010). Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): Its relevance for acute and chronic pain states. Current Opinion in Anesthesiology,23(5), 611–615.
Acknowledgments
This research was supported by a Grant from Fondation Apicil and by a Grant from Fondation de France. The authors wish to sincerely thank the adults who accepted to participate in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author declares that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dubois, A., Boudjarane, M., Le Fur-Bonnabesse, A. et al. Pain Modulation Mechanisms in ASD Adults. J Autism Dev Disord 50, 2931–2940 (2020). https://doi.org/10.1007/s10803-019-04361-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-019-04361-x