Abstract
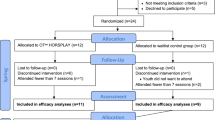
In this study the effectiveness of an equine-assisted therapy (EAT) in improving adaptive and executive functioning in children with autism spectrum disorder (ASD) was examined (children attending EAT, n = 15, control group n = 13; inclusion criteria: IQ > 70). Therapeutic sessions consisted in structured activities involving horses and included both work on the ground and riding. Results indicate an improvement in social functioning in the group attending EAT (compared to the control group) and a milder effect on motor abilities. Improved executive functioning was also observed (i.e. reduced planning time in a problem-solving task) at the end of the EAT program. Our findings provide further support for the use of animal-assisted intervention programs as complementary intervention strategies for children with ASD.


Similar content being viewed by others
References
All, A. C., Loving, G. L., & Crane, L. L. (1999). Animals, horseback riding, and implications for rehabilitation therapy. Journal of Rehabilitation, 65(3), 49–57.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-V). Arlington, VA: American Psychiatric Publishing.
Bass, M. M., Duchowny, C. A., & Llabre, M. M. (2009). The effect of therapeutic horseback riding on social functioning in children with autism. Journal of Autism and Developmental Disorders, 39, 1261–1267.
Berry, A., Borgi, M., Francia, N., Alleva, E., & Cirulli, F. (2013). Use of assistance and therapy dogs for children with autism spectrum disorders: A critical review of the current evidence. Journal of Alternative and Complementary Medicine, 19(2), 73–80.
Bizub, A. L., Joy, A., & Davidson, L. (2003). “It’s like being in another world”: Demonstrating the benefits of therapeutic horseback riding for individuals with psychiatric disability. Psychiatric Rehabilitation Journal, 26(4), 377–384.
Borgi, M., & Cirulli, F. (2015). Attitudes toward animals among kindergarten children: Species preferences. Anthrozoos, 28(1), 45–59.
Borgi, M., Cogliati-Dezza, I., Brelsford, V., Meints, K., & Cirulli, F. (2014). Baby schema in human and animal faces induces cuteness perception and gaze allocation in children. Frontiers in Psychology, 5, 411.
Bronson, C., Brewerton, K., Ong, J., Palanca, C., & Sullivan, S. J. (2010). Does hippotherapy improve balance in persons with multiple sclerosis: A systematic review. European Journal of Physical and Rehabilitation Medicine, 46, 347–353.
Cerino, S., Cirulli, F., Chiarotti, F., & Seripa, S. (2011). Non conventional psychiatric rehabilitation in schizophrenia using therapeutic riding: The FISE multicentre Pindar project. Annali dell Istituto Superiore di Sanita, 47(4), 409–414.
Cerino, S., & Frascarelli, M. (Eds.). (2011). Testo guida di riabilitazione equestre. Roma: Federazione Italiana Sport Equestri.
Christon, L., Mackintosh, V., & Myers, B. (2010). Use of complementary and alternative medicine (CAM) treatments by parents of children with autism spectrum disorders. Research in Autism Spectrum Disorders, 4(2), 249–259.
Cirulli, F., Borgi, M., Berry, A., Francia, N., & Alleva, E. (2011). Animal-assisted interventions as innovative tools for mental health. Annali dell Istituto Superiore di Sanita, 47(4), 341–348.
Endenburg, N., & van Lith, H. A. (2011). The influence of animals on the development of children. The Veterinary Journal, 190(2), 208–214.
Freund, L. S., Brown, O. J., & Huff, P. R. (2011). Equine-assisted activities and therapy for individuals with physical and developmental disabilities: An overview of research findings and the types of research currently being conducted. In P. McCardle, S. McCune, J. A. Griffin, L. Esposito, & L. S. Freund (Eds.), Animals in our lives: Human animal interaction in family, community and therapeutic settings. Baltimore: Paul H. Brookes.
Gabriels, R., Agnew, J., Holt, K., Shoffner, A., Zhaoxing, P., & Ruzzano, S. (2012). Pilot study measuring the effects of therapeutic horseback riding on school-age children and adolescents with autism spectrum disorders. Research in Autism Spectrum Disorders, 6(2), 578–588.
Gee, N. R. (2011). The role of pets in the classroom. In S. M. P. McCardle, J. A. Griffin, L. Esposito, & L. Freund (Eds.), Animals in our lives: Human–animal interaction in family, community, and therapeutic settings. Baltimore, MD: Brookes Publishing.
Geschwind, D. H., & Levitt, P. (2007). Autism spectrum disorders: Developmental disconnection syndromes. Current Opinion in Neurobiology, 17(1), 103–111.
Gowen, E., & Hamilton, A. (2013). Motor abilities in autism: A review using a computational context. Journal of Autism and Developmental Disorders, 43(2), 323–344.
Holm, M. B., Baird, J. M., Kim, Y. J., Rajora, K. B., D’Silva, D., Podolinsky, L., et al. (2014). Therapeutic horseback riding outcomes of parent-identified goals for children with autism spectrum disorder: An ABA’ multiple case design examining dosing and generalization to the home and community. Journal of Autism and Developmental Disorders, 44(4), 937–947.
Keino, H., Funahashi, A., Keino, H., Miwa, C., Hosokawa, M., & Hayashi, Y. (2009). Psycho-educational horseback riding to facilitate communication ability of children with pervasive developmental disorders. Journal of Equine Science, 20(4), 79–88.
Kern, J. K., Fletcher, C. L., Garver, C. R., Mehta, J. A., Grannemann, B. D., Knox, K. R., et al. (2011). Prospective trial of equine-assisted activities in autism spectrum disorder. Alternative Therapies in Health and Medicine, 17(3), 14–20.
Lanning, B. A., Baier, M. E., Ivey-Hatz, J., Krenek, N., & Tubbs, J. D. (2014). Effects of equine assisted activities on autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(8), 1897–1907.
Lechner, H. E., Kakebeeke, T. H., Hegemann, D., & Baumberger, M. (2007). The effect of hippotherapy on spasticity and on mental well-being of persons with spinal cord injury. Archives of Physical Medicine and Rehabilitation, 88(10), 1241–1248.
Marino, L. (2012). Construct validity of animal-assisted therapy and activities: How important is the animal in AAT? Anthrozoos, 25(3), 139–151.
McCardle, P., McCune, S., Griffin, J.A., & Maholmes, V. (Eds.). (2011). How animals affect us: Examining the influences of human–animal interaction on child development and human health. Washington, DC: American Psychological Association.
McNicholas, J., & Collis, G. M. (2000). Dogs as catalysts for social interactions: Robustness of the effect. British Journal of Psychology, 91, 61–70.
Memishevikj, H., & Hodzhikj, S. (2010). The effects of equine-assisted therapy in improving the psychosocial functioning of children with autism. Journal of Special Education and Rehabilitation, 11(3–4), 57–67.
Munoz-Lasa, S., Ferriero, G., Valero, R., Gomez-Muniz, F., Rabini, A., & Varela, E. (2011). Effect of therapeutic horseback riding on balance and gait of people with multiple sclerosis. Giornale Italiano Di Medicina Del Lavoro Ed Ergonomia, 33(4), 462–467.
O’Haire, M. E. (2013). Animal-assisted intervention for autism spectrum disorder: A systematic literature review. Journal of Autism and Developmental Disorders, 43(7), 1606–1622.
Reichow, B. (2012). Overview of meta-analyses on early intensive behavioral intervention for young children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 42(4), 512–520.
Rogers, S. J., & Vismara, L. A. (2008). Evidence-based comprehensive treatments for early autism. Journal of Clinical Child & Adolescent Psychology, 37(1), 8–38.
Schultz, P. N., Remick-Barlow, G. A., & Robbins, L. (2007). Equine-assisted psychotherapy: A mental health promotion/intervention modality for children who have experienced intra-family violence. Health and Social Care in the Community, 15(3), 265–271.
Shallice, T. (1982). Specific impairments of planning. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 298(1089), 199–209.
Snider, L., Korner-Bitensky, N., Kammann, C., Warner, S., & Saleh, M. (2007). Horseback riding as therapy for children with cerebral palsy: Is there evidence of its effectiveness? Physical & Occupational Theraphy in Pediatrics, 27(2), 5–23.
Sparrow, S., Balla, D., & Cicchetti, D. (1984). The Vineland adaptive behavior scales: Interview edition, survey form manual. Circle Pines, MN: American Guidance Service.
Thomas, K. C., Morrissey, J. P., & McLaurin, C. (2007). Use of autism-related services by families and children. Journal of Autism and Developmental Disorders, 37(5), 818–829.
Tseng, S. H., Chen, H. C., & Tam, K. W. (2013). Systematic review and meta-analysis of the effect of equine assisted activities and therapies on gross motor outcome in children with cerebral palsy. Disability and Rehabilitation, 35(2), 89–99.
Umbarger, G. T. (2007). State of the evidence regarding complimentary and alternative medical treatments for autism spectrum disorders. Education & Training in Developmental Disabilities, 42(4), 437–447.
Vismara, L. A., & Rogers, S. J. (2010). Behavioral treatments in autism spectrum disorder: What do we know? Annual Review of Clinical Psychology, 6, 447–468.
Ward, S. C., Whalon, K., Rusnak, K., Wendell, K., & Paschall, N. (2013). The association between therapeutic horseback riding and the social communication and sensory reactions of children with autism. Journal of Autism and Developmental Disorders, 43(9), 2190–2198.
Warren, Z., McPheeters, M. L., Sathe, N., Foss-Feig, J. H., Glasser, A., & Veenstra-Vanderweele, J. (2011). A systematic review of early intensive intervention for autism spectrum disorders. Pediatrics, 127(5), e1303–e1311.
Wechsler, D. (1991). Wechsler Intelligence Scale for children-third edition. San Antonio, TX: The Psychological Corporation.
Whalen, C. N., & Case-Smith, J. (2012). Therapeutic effects of horseback riding therapy on gross motor function in children with cerebral palsy: A systematic review. Physical & Occupational Therapy in Pediatrics, 32(3), 229–242.
Winchester, P., Kendall, K., Peters, H., Sears, N., & Winkley, T. (2002). The effect of therapeutic horseback riding on gross motor function and gait speed in children who are developmentally delayed. Physical & Occupational Therapy in Pediatrics, 22(3–4), 37–50.
Wood, L., Giles-Corti, B., & Bulsara, M. (2005). The pet connection: Pets as a conduit for social capital? Social Science and Medicine, 61(6), 1159–1173.
Wuang, Y. P., Wang, C. C., Huang, M. H., & Su, C. Y. (2010). The effectiveness of simulated developmental horse-riding program in children with autism. Adapted Physical Activity Quarterly, 27(2), 113–126.
Zadnikar, M., & Kastrin, A. (2011). Effects of hippotherapy and therapeutic horseback riding on postural control or balance in children with cerebral palsy: A meta-analysis. Developmental Medicine and Child Neurology, 53(8), 684–691.
Acknowledgments
We would like to thank the Department of Equestrian Rehabilitation of the Italian Equestrian Federation (Federazione Italiana Sport Equestri, FISE) for their generous support; Lino Cavedon, Luca Farina (Scientific Director), National Centre for the Animal-Assisted Intervention (Centro di Referenza Nazionale per gli Interventi Assistiti con gli Animali, Istituto Zooprofilattico Sperimentale delle Venezie), Claudia Cerulli and Attilio Parisi (IUSM, Rome), and Stefano Seripa (DSM ASL ROMA F) for their precious advices during study design; Maddalena Insogna and Giada Reali for their support during data analysis; Daniela Zoppi and Antonella Piciullo (Centro di Riabilitazione Equestre, Villa Buon Respiro, Viterbo) for the drawings used during therapeutic sessions.
Author Contributions
MBo, FCi, SC conceived of the study, participated in its design and coordination and drafted the manuscript; DL, AV participated in the coordination of the study and helped to draft the manuscript; FCh participated in the design of the study, performed the statistical analysis and helped to draft the manuscript; MFr participated in the design and coordination of the study; MBr, EN, MM participated in the design and interpretation of the data and performed the measurements; CV, CDS, FB, MFa performed the measurements: All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borgi, M., Loliva, D., Cerino, S. et al. Effectiveness of a Standardized Equine-Assisted Therapy Program for Children with Autism Spectrum Disorder. J Autism Dev Disord 46, 1–9 (2016). https://doi.org/10.1007/s10803-015-2530-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-015-2530-6