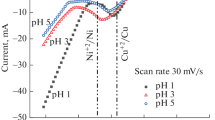
Abstract
This work aims to electrodeposit Cr from a solution containing only Cr3+ ions but not any other complexing, buffering and pH agents, and study its corrosion behavior. The solution was investigated by cyclic voltammetry and ultraviolet–visible spectroscopy. It was obtained that it is rich in native Cr complexes including Cl. Cr was successfully coated from this solution by binary potential loop electrodeposition. First, − 0.7 V potential was applied for a time interval ranging from 5 to 30 s. Then, a − 1.8 V potential was applied to deposit the Cr layer with the thickness changed from 50 to 1000 nm. X-ray diffraction patterns showed that all films are in a bcc crystal structure. The Fourier-transform infrared spectroscopy, X-ray photoelectron spectroscopy and optical profilometry measurements inferred that the coatings include hydrated-Cl complexes mostly edges of the coating and cracks. The scanning electron microscopy images indicated that the cracks on the surface are tiny for the layers thinner than 500 nm but prominent for the layers thicker than 500 nm. The corrosion behavior was examined by electrochemical impedance spectroscopy and Tafel measurements. The coatings electrodeposited by the binary potential loop had a corrosion rate lower and resistance higher than those of the coatings electrodeposited at − 1.8 V potential. The corrosion parameters became superior for the coatings with layers thinner than 500 nm and the aged ones.
Graphical abstract










Similar content being viewed by others
References
Lindsay JH (1997) Decorative and hard chromium plating. Plat Surf Finish 84:50–51
Leimbach M, Tschaar C, Schmidt U, Bund A (2018) Electrochemical characterization of chromium deposition from trivalent solution for decorative application by EQCM and near surface pH measurements. Electrochim Acta 270:104–109. https://doi.org/10.1016/j.electacta.2018.03.011
Zhao H, Lui W, Li Q, Zhang B, Jianguo L, Yan C, Liu C (2020) Mechanism of chromium electrodeposition from Cr(III) baths on nickel and chromium electrode surfaces. Int J Electrochem Sci 15:8979–8989. https://doi.org/10.20964/2020.09.23
Vaiopoulou E, Gikas P (2020) Regulations for chromium emissions to the aquatic environment in Europe and elsewhere. Chemosphere 254:126876. https://doi.org/10.1016/j.chemosphere.2020.12
Hilali N, Mohammadi H, Amine A, Zine N, Errachid A (2020) Recent advances in electrochemical monitoring of chromium. Sensors 20(18):5153. https://doi.org/10.3390/s20185153
Saillard R, Viguier B, Odemer G, Pugliara A, Fori B, Blanc C (2018) Influence of the microstructure on the corrosion behaviour of 2024 aluminium alloy coated with a trivalent chromium conversion layer. Corros Sci 142:132–199. https://doi.org/10.1016/j.corsci.2018.07.007
Quan C, He Y (2015) Properties of nanocrystalline Cr coatings prepared by cathode plasma electrolytic deposition from trivalent chromium electrolyte. Surf Coat Technol 269:319–323. https://doi.org/10.1016/j.surfcoat.2015.02.001
Huang CA, Chang JH, Chen CY, Liao KY, Mayer J (2013) Microstructure and electrochemical corrosion behavior of Cr-Ni-Fe alloy deposits electroplated in the presence of trivalent Cr ions. Thin Solid Films 544:69–73. https://doi.org/10.1016/j.tsf.2013.04.124
Giovanardi R, Orlando G (2011) Chromium electrodeposition from Cr(III) aqueous solutions. Surf Coat Technol 205:3947–3955. https://doi.org/10.1016/j.surfcoat.2011.02.027
Protsenko VS, Danilov FI (2014) Chromium electroplating from trivalent chromium baths as an environmentally friendly alternative to hazardous hexavalent chromium baths: comparative study on advantages and disadvantages. Clean Technol Environ Policy 16:1201–1206. https://doi.org/10.1007/s10098-014-0711-1
Liang A, Li Y, Liang H, Ni L, Zhang J (2017) A favorable chromium coating electrodeposited from Cr(III) electrolyte reveals anti-wear performance similar to conventional hard chromium. Mater Lett 189:221–224. https://doi.org/10.1016/j.matlet.2016.12.022
Xu L, Pi L, Dou Y, Cui Y, Mao X, Lin A, Fernandez C, Peng C (2020) Electroplating of thick hard chromium coating from a trivalent chromium bath containing ternary complexing agent: a methodological and mechanistic study. ACS Sustain Chem Eng 8:15540–15549. https://doi.org/10.1021/acssuschemeng.0c04529
Hong G, Siow KS, Zhiqiang G, Hsieh AK (2001) Hard chromium plating from trivalent chromium solution. Plat Surf Finish 88:69–73
Protsenko VS (2014) Electrodeposition from trivalent chromium baths as an environmentally friendly alternative to electroplating from hazardous hexavalent chromium baths. ChemXpress 4:246–252
Matsumoto K, Zhang J, Yoneda N, Numata K, Okuno K, Hagiwara R (2022) Concentrated aqueous solution of chromium dichloride for chromium metal electrodeposition. J Phys Chem C 126:14346–14352. https://doi.org/10.1021/acs.jpcc.2c04715
Buker L, Bottcher R, Leimbach M, Hahne T, Dickbreder R, Bund A (2022) Influence of carboxylic acids on the performance of trivalent chromium electrolytes for the deposition of functional coatings. Electrochim Acta 411:140054. https://doi.org/10.1016/j.electacta.2022.140054
Mahdavi S, Allahkaram SR, Heidarzadeh A (2018) Characteristics and properties of Cr coatings electrodeposited from Cr(III) baths. Mater Res Express. https://doi.org/10.1088/2053-1591/aaeb4f
Xu D, Ni S, Bu Y, Liu J, Ling G, Wang H (2023) Electrodeposition of high-quality Cr coatings with solid solution Al from Cr2+ electrolyte. Surf Coat Technol 425:129121. https://doi.org/10.1016/j.surfcoat.2022.129121
Song Y, Chin DT (2000) Pulse plating of hard chromium from trivalent baths. Plat Surf Finish 87:80–87
Conde DF, Gamboa GO, Gonzalez JT (2020) Study on the electrodeposition of chromium from Cr(III) solution in the presence of oxalate and acetate anions. Int J Electrochem Sci 15:5741–5757. https://doi.org/10.20964/2020.06.70
Del Pianta D, Frayret J, Gleyzes C, Cugnet C, Dupin JC, Le Hecho I (2018) Determination of the chromium(III) reduction mechanism during chromium electroplating. Electrochim Acta 284:234–241. https://doi.org/10.1016/j.electacta.2018.07.114
Haque E, Haoque A, Islam M, Islam S, Mustafa M (2017) Effect of various operating parameters on trivalent chromium electroplating. J Sci Research Rep 13:1–9 (Article, no. JSRR.31411 ISSN: 2320-0227)
Zeng Z, Zhang Y, Zhao W, Zhang J (2011) Role of complexing ligands in trivalent chromium electrodeposition. Surf Coat Technol 205:4771–4775. https://doi.org/10.1016/j.surfcoat.2011.04.019
Saravanan G, Mohan S (2010) Structure, current efficiency, and corrosion properties of brush electrodeposited (BED) Cr from Cr(III) dimethy formamide (DMF)-Bath. J Appl Electrochem 40:1–6. https://doi.org/10.1007/s10800-009-9962-7
Kumar UP, Kennady CJ (2015) Characterization of chromium electrodeposits obtained from trivalent electrolytes containing formaldehyde as additive. Int J Thin Films Sci Technol 4:147–153. https://doi.org/10.12785/ijtfst/040213
Lu CE, Pu N, Hou KH, Tseng CC, Ger MD (2013) The effect of formic acid concentration on the conductivity and corrosion resistance of chromium carbide coatings electroplated with trivalent chromium. Appl Surf Sci 282:544–551. https://doi.org/10.1016/j.apsusc.2013.06.008
Protsenko VS, Gordiienko VO, Danilov FI, Kwon SC (2011) Thick chromium electrodeposition from trivalent chromium bath containging carbamide and formic acid. Metal Finish 109:33–37. https://doi.org/10.1016/S0026-0576(11)80066-8
Suarez O, Olaya JJ, Suarez MF, Rodil SE (2012) Corrosion resistance of decorative chromium films obtained from trivalent chromium solutions. J Chil Chem Soc 57:977–982. https://doi.org/10.4067/s0717-97072012000100005
Sziráki L, Kuzmann E, Papp K, Chisholm CU, El-Sharif MR, Havancsák K (2012) Electrochemical behaviour of amorphous electrodeposited chromium coatings. Mater Chem Phys 133:1092–1100. https://doi.org/10.1016/j.matchemphys.2012.02.021
Mehdipour N, Rezaei M, Mahidashti Z (2020) Influence of glycine additive on corrosion and wear performance of electroplated trivalent chromium coating. Int J Min Met Mater 27:544–554. https://doi.org/10.1007/s12613-020-1975-6
Benaben P (2011) An overview of hard chromium plating using trivalent chromium solutions. Plat Surf Finish 98:7–14
Chien CW, Liu CL, Chen FJ, Lin KH, Lin CS (2012) Microstructure and properties of carbon sulfur-containing chromium deposits electroplated in trivalent chromium baths with thiosalicylicacid. Electrochim Acta 72:74–80. https://doi.org/10.1016/j.electacta.2012.03.168
Molodkina EB, Ehrenburg MR, Broekmann P, Rudnev AV (2020) Electrodeposition of chromium on single-crystal electrodes from solutions of Cr(II) and Cr(III) salts in ionic liquids. J Electroanal Chem 860:113892. https://doi.org/10.1016/j.jelechem.2020.113892
Martinuzzi MS, Donati L, Giurlani W, Pizzetti F, Galvanetto E, Calisi N, Innocenti M, Caporali S (2022) A comparative research on corrosion behavior of electroplated and magnetron sputtered chromium coatings. Coatings 12:257. https://doi.org/10.3390/coatings12020257
Zhang S, Li Y, Wang C, Zhao X (2018) Preparation of a Cr coating on low-carbon steel by electrodeposition in a NaCl-KCl-NaF-Cr2O3 molten salt. Int J Electrochem Sci 14:91–101. https://doi.org/10.20964/2019.01.07
Survilienė S, Eugénio S, Vilar R (2011) Chromium electrodeposition from [BMIm][BF4] ionic liquid. J Appl Electrochem 41:107–114. https://doi.org/10.1007/s10800-010-0218-3
Protsenko VS, Bobrova LS, Danilov FI (2021) Effects of water and sodium dodecyl sulfate additives on Cr(III) ions electroreduction in a deep eutectic solvent. Vopr Khimii i Khimicheskoi Tekhnol 2:110–116
Khani H, Brennecke JF (2019) Hard chromium composite electroplating on high-strength stainless steel from a Cr(III)-ionic liquid solution. Electrochem Commun 107:106537. https://doi.org/10.1016/j.elecom.2019.106537
Chandrasekar MS, Pushpavanam M (2008) Pulse and pulse reverse plating-conceptual, advantages and applications. Electrochim Acta 53:3313–3322. https://doi.org/10.1016/j.electacta.2007.11.054
Imaz N, García-Lecina E, Díez JA, Ostra M, Sarret M (2012) Chemometrics applied to functional chromium electroplating by pulse plating techniques. Trans Inst Met Finish 90:259–266. https://doi.org/10.1179/0020296712z.00000000046
Yeo S, Kim JH, Yun HS (2020) Effect of pulse current and coating thickness on the microstructure and FCCI resistance of electroplated chromium on HT9 steel cladding. Surf Coat Technol 389:125652. https://doi.org/10.1016/j.surfcoat.2020.125652
Ahmedi K, Dole N, Karadavut O, Robles Hernandez FC, Hall TD, Taylor EJ, Brankovic SR (2022) Crack free Cr coatings from Cr3+ electrolyte. J Electrochem Soc 169:012504. https://doi.org/10.1149/1945-7111/ac4bfa
Imaz N, Ostra M, Vidal M, Díez JA, Sarret M, García-Lecina E (2014) Corrosion behaviour of chromium coatings obtained by direct and reverse pulse plating electrodeposition in NaCl aqueous solution. Corros Sci 78:251–259. https://doi.org/10.1016/j.corsci.2013.10.005
Kuznetsov VV, Vinokurov EG, Kudryavtsev VN (2001) Kinetics of electroreduction of Cr3+ ions in sulfate solutions. Russ J Electrochem 37(7):699–703. https://doi.org/10.1023/a:1016712616902
Protsenko V (2023) Kinetics and mechanism of electrochemical reactions occurring during the chromium electrodeposition from electrolytes based on Cr(III) compounds: a literature review. Reactions 4(3):398–419. https://doi.org/10.3390/reactions4030024
Cullity BD (1978) Elements of X-ray diffraction, 2nd edn. Addison-Wesley, London
Vicenzo A, Cavallotti PL (2004) Growth modes of electrodeposited cobalt. Electrochim Acta 49:4079–4089. https://doi.org/10.1016/j.electacta.2004.04.001
Stern M, Geary AL (1957) Electrochemical polarization. I. A theoretical analysis of the shape of polarization curve. J Electrochem Soc 104:56–63. https://doi.org/10.1149/1.2428496
Stern M (1957) Electrochemical polarization. J Electrochem Soc 104:559. https://doi.org/10.1149/1.2428653
Johnson DA (1985) Chemical and electrochemical behavior of the Cr(III)/Cr(II) half-cell in the iron-chromium redox energy storage system. J Electrochem Soc 132:1053
Danilov FI, Velichenko AB (1993) Electrocatalytic activity of anodes in reference to Cr(III) oxidation reaction. Electrochim Acta 38:437–440. https://doi.org/10.1016/0013-4686(93)85162-r
Pletcher D, Tait SJD (1981) The electrolytic oxidation of chromic sulphate to chromic acid: the effects of lead anode preparation. J Appl Electrochem 11(4):493–502. https://doi.org/10.1007/bf01132438
He X, Li C, Zhu Q, Hou B, Jiang Y, Wu L (2014) Electrochemical mechanism of Cr(III) reduction for preparing crystalline chromium coatings based on 1-butyl-3-methylimidazolium hydrogen sulfate ionic liquid. RSC Adv 4:64174–64182. https://doi.org/10.1039/c4ra12335b
Niu Y, Zhang S, Cheng Q, Zhang Z, Yao Z, Moliar O (2020) Characterization and corrosion resistance study of the Fe-Cr films electrodeposited from trivalent chromium sulfate electrolyte. Mater Res Express 6:126430. https://doi.org/10.1088/2053-1591/ab5538
Protsenko VS, Bobrova LS, Baskevich S, Korniy SA, Danilov FU (2018) Electrodeposition of chromium coatings from a choline chloride based ionoc liquid with addition of water. J Chem Technol Metall 53:906–915
Protsenko V, Danilov F (2009) Kinetics and mechanism of chromium electrodeposition from formate and oxalate solutions of Cr(III) compounds. Electrochim Acta 54(24):5666–5672. https://doi.org/10.1016/j.electacta.2009.04.072
Weaver M, Anson F (1976) Distinguishing between inter and outer sphere electrode reactions. Reactivity patterns for some Chromium(III)–Chromium(II) electron-transfer reactions at mercury electrodes. Inorg Chem 15:1871–1880
Sutin N (1966) The kinetics of inorganic reactions in solution. Annu Rev Phys Chem 17(1):119–172
Mandich NV (1997) Chemistry and theory of chromium deposition: part 1-chemistry. Plat Surf Finish 84(5):108–115
El Rehim A, Sayed S, Ibrahim MAM, Dankeria MM (2002) Thin films of chromium electrodeposition from a trivalent chromium electrolyte. Trans IMF 80(1):29–33. https://doi.org/10.1080/00202967.2002.11871424
Phuong NV, Kwon SC, Lee JY, Shin J, Huy BT, Lee YI (2011) Mechanistic study on the effect of PEG molecules in a trivalent chromium electrodeposition process. Microchem J 99(1):7–14. https://doi.org/10.1016/j.microc.2011.02.017
Liang A, Ni L, Liu Q, Zhang J (2013) Structure characterization and tribological properties of thick chromium coating electrodeposited from a Cr(III) electrolyte. Surf Coat Technol 218:23–29. https://doi.org/10.1016/j.surfcoat.2012.12.021
Haciismailoglu M, Alper M (2011) Effect of electrolyte pH and Cu concentration on microstructure of electrodeposited Ni-Cu alloy films. Surf Coat Technol 206:1431438. https://doi.org/10.1016/j.surfcoat.2011.09.0
Jain S, Shah J, Negi NS, Sharma C, Kotnala RK (2019) Significance of interface barrier at electrode of hematite hydroelectric cell for generating ecopower by water splitting. Int J Energy Res 43(9):4743–4755. https://doi.org/10.1002/er.4613
Biesinger MC, Brown C, Mycroft JR, Davidson RD, McIntyre NS (2004) X-ray photoelectron spectroscopy studies of chromium compounds. Surf Interface Anal 36(12):1550–1563. https://doi.org/10.1002/sia.1983
McCafferty E, Wightman JP (1998) Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf Interface Analy 26(8):549–564. https://doi.org/10.1002/(sici)1096-9918(199807)26:8%3c549::aid-sia396%3e3.0.co;2-q
Papavinasam S (2021) Electrochemical polarization techniques for corrosion monitoring. In: Yang L (ed) Techniques for Corrosion Monitoring, Woodhead Publishing Series in Metals and Surface Engineering, 2nd edn. Woodhead Publishing, pp. 45-77. https://doi.org/10.1016/B978-0-08-103003-5.00003-5
Acknowledgements
E. Kus is thankful to the TUBITAK for the scholarship of Tubitak-2244. This work was supported by Uludag University under Grant no. FDK-2022-1262, FAY-2022-811 and UAP(F)-2010/56, and by TAI under Grant no. Ar-Ge-21-044. Special thanks to Dr. M. Cuneyt Hacıismailoglu for the UV–Vis spectroscopy measurements. SEM-EDX measurements were performed at Bursa Technical University.
Author information
Authors and Affiliations
Contributions
All authors have contribution in this work. Ms .Esra Kus was a Ph.D. student under the supervision of Dr. Murside Haciismailoglu and this manuscript is an output of her thesis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kus, E., Haciismailoglu, M. & Alper, M. Binary potential loop electrodeposition and corrosion resistance of Cr coatings. J Appl Electrochem (2024). https://doi.org/10.1007/s10800-024-02135-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10800-024-02135-7