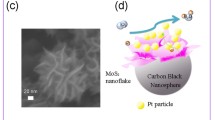
Abstract
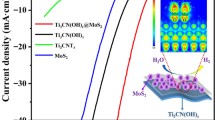
Search for a Platinum metal-free catalyst for Proton Exchange Membrane Fuel Cells (PEMFCs) is a concern to scientists as the Pt-catalyzed electrode comprises about 45% of the entire fuel cell stack cost. Due to layered structure, Two-dimensional (2D) MoS2 nanostructured materials have recently drawn attention as effective catalysts for oxygen reduction reaction (ORR). The 1T-phase of MoS2 (1T-MoS2) has higher electronic conductivity than the 2H-phase (2H-MoS2); high surface area 1T-MoS2 can have high electrocatalytic activity towards PEFFCs. Here, we report hydrothermally synthesized 2H-MoS2 and solvothermally synthesized mixed 1T/2H-MoS2 phases as a catalyst for ORR in PEM Fuel Cells. Owing to the high BET-specific surface area, hybrid 1T/2H-MoS2 showed better ORR activity than 2H-MoS2. The limiting current density of the 1T/2H-MoS2 hybrid structure is ‒7.1 mA cm−2 compared to Pt/C (‒8.2 mA cm−2) at 1600 rpm. The Tafel slope values of 2H-MoS2, 1T/2H-MoS2, and Pt/C are 92.7 mV dec−1, 57.5 mV dec−1, and 39.3 mV dec−1, respectively. The electron transferred during ORR are 3.8 and 1.8, respectively, for 1T/2H-MoS2 and 2H-MoS2 (in 0.1 M KOH) ; suggesting less formation of undesirable peroxide formation than 2H-MoS2. The 1T/2H-MoS2 displays better electrochemical durability compared to 2H-MoS2 (after 2000 CV cycles).
Graphical abstract








Similar content being viewed by others
References
Holton OT, Stevenson JW (2013) The role of platinum in proton exchange membrane fuel cells evaluation of platinum’s unique properties for use in both the anode and cathode of a proton exchange membrane fuel cell. Platinum Met Rev 57:259–271
Martinez U, Komini Babu S, Holby EF et al (2019) Progress in the Development of Fe-Based PGM-Free Electrocatalysts for the Oxygen reduction reaction. Adv Mater 31:1–20. https://doi.org/10.1002/adma.201806545
Raj CR, Samanta A, Noh SH et al (2016) Emerging new generation electrocatalysts for the oxygen reduction reaction. J Mater Chem A 4:11156–11178. https://doi.org/10.1039/c6ta03300h
Samad S, Loh KS, Yin Wong W, Lee TK, Sunarso J STC and. D (2018) Carbon and non-carbon support materials for platinum-based catalysts in fuel cells. Int J Hydrogen Energy 43:7823–7854. https://doi.org/10.1016/j.ijhydene.2018.02.154
Choi W, Choudhary N, Han GH et al (2017) Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater Today 20:116–130. https://doi.org/10.1016/j.mattod.2016.10.002
Alinejadian N, Kollo L, Odnevall I (2022) Progress in additive manufacturing of MoS2-based structures for energy storage applications – A review. Mater Sci Semicond Process 139:106331. https://doi.org/10.1016/j.mssp.2021.106331
Huang Y, Guo J, Kang Y et al (2015) Two dimensional atomically thin MoS2 nanosheets and their sensing applications. Nanoscale 7:19358–19376. https://doi.org/10.1039/c5nr06144j
Shrivastav M, Kushwaha HS, Dhiman R (2022) 2H-MoS2 as an electrode material for oxygen reduction reaction and supercapacitor applications. Mater Today. https://doi.org/10.1016/j.matpr.2022.10.202
Zhou X, Xu B, Lin Z et al (2014) Hydrothermal synthesis of flower-like MoS2nanospheres for electrochemical supercapacitors. J Nanosci Nanotechnol 14:7250–7254. https://doi.org/10.1166/jnn.2014.8929
Krishnan U, Kaur M, Singh K et al (2019) A synoptic review of MoS 2: synthesis to applications. Superlattices Microstruct 128:274–297. https://doi.org/10.1016/j.spmi.2019.02.005
Gupta D, Chauhan V, Kumar R (2020) A comprehensive review on synthesis and applications of molybdenum disulfide (MoS2) material: past and recent developments. Inorg Chem Commun 121:108200. https://doi.org/10.1016/j.inoche.2020.108200
Wang H, Li C, Fang P et al (2018) Synthesis, properties, and optoelectronic applications of two-dimensional MoS2 and MoS2-based heterostructures. Chem Soc Rev 47:6101–6127. https://doi.org/10.1039/c8cs00314a
Habibi Jetani G, Rahmani MB (2022) Exploring the effect of hydrothermal precursor pH on the photosensitivity of 1T/2H–MoS2 nanosheets. Opt Mater (Amst) 124:111974. https://doi.org/10.1016/j.optmat.2022.111974
Chen Z (2016) Small dopants make big differences: enhanced Electrocatalytic performance of MoS2 monolayer for Oxygen Reduction reaction (ORR) by N – and P – doping. Electrochim Acta. https://doi.org/10.1016/j.electacta.2016.12.144
Suresh C, Mutyala S, Mathiyarasu J (2016) Support interactive synthesis of nanostructured MoS 2 electrocatalyst for oxygen reduction reaction. Mater Lett 164:417–420. https://doi.org/10.1016/j.matlet.2015.11.052
Hu Y, Chua DHC (2016) Synthesizing 2D MoS2 nanofins on carbon nanospheres as catalyst support for Proton Exchange Membrane Fuel Cells. Sci Rep 6:1–10. https://doi.org/10.1038/srep28088
Jin Q, Liu N, Chen B, Mei D (2018) Mechanisms of semiconducting 2H to metallic 1T phase transition in two-dimensional MoS 2 nanosheets. J Phys Chem C 122:28215–28224. https://doi.org/10.1021/acs.jpcc.8b10256
Gan X, Lee LYS, Wong KY et al (2018) 2H/1T phase transition of Multilayer MoS2 by Electrochemical Incorporation of S Vacancies. ACS Appl Energy Mater 1:4754–4765. https://doi.org/10.1021/acsaem.8b00875
Taufik A, Asakura Y, Kato H et al (2020) 1T/2H-MoS2 engineered by in-situ ethylene glycol intercalation for improved toluene sensing response at room temperature. Adv Powder Technol 31:1868–1878. https://doi.org/10.1016/j.apt.2020.02.022
Thi Xuyen N, Ting JM (2017) Hybridized 1T/2H MoS2 having controlled 1T concentrations and its use in Supercapacitors. Chem - A Eur J 23:17348–17355. https://doi.org/10.1002/chem.201703690
Wang D, Zhang X, Bao S et al (2017) Phase engineering of a multiphasic 1T/2H MoS2 catalyst for highly efficient hydrogen evolution. J Mater Chem A 5:2681–2688. https://doi.org/10.1039/c6ta09409k
Dhiman R, Stamatin SN, Andersen SM et al (2013) Oxygen reduction and methanol oxidation behaviour of SiC based Pt nanocatalysts for proton exchange membrane fuel cells. J Mater Chem A 1:15509–15516. https://doi.org/10.1039/c3ta12744c
Online VA, Li H, Xie F et al (2016) Preparation and adsorption capacity of porous MoS2 nanosheets. RSC Adv 6:105222–105230. https://doi.org/10.1039/C6RA22414H
Li Y, Chang K, Sun E, Shangguan H, Tang B, Li JS and ZC (2020) Selective Preparation of 1T- and 2H-Phase MoS2 nanosheets with abundant monolayer structure and their applications in Energy Storage devices. ACS Appl Energy Mater 3:998–1009. https://doi.org/10.1021/acsaem.9b02043
Wen YN, Xia MG, Zhang SL (2018) Structural and magnetic properties of MoS2 monolayer zigzag nanoribbon doped by Ti, V, Cr, and Mn. Phys Lett A 382:2354–2360. https://doi.org/10.1016/j.physleta.2018.05.050
Jagminas A, Niaura G, Žalneravičius R et al (2016) Laser light induced transformation of molybdenum disulphide-based nanoplatelet arrays. Sci Rep 6:2–10. https://doi.org/10.1038/srep37514
Yougui Liao (2016) Energy peak overlapping in EDS Spectrum. In: Practical Electron Microscopy and Database
Wu M, Zhan J, Wu K et al (2017) Metallic 1T MoS2 nanosheet arrays vertically grown on activated carbon fiber cloth for enhanced Li-ion storage performance. J Mater Chem A. https://doi.org/10.1039/C7TA03497K
Huang S, You Z, Jiang Y et al (2020) Fabrication of ultrathin MoS2 nanosheets and application on adsorption of organic pollutants and heavy metals. Processes 8:1–17. https://doi.org/10.3390/PR8050504
Dhiman R (2021) Investigation of cathode electrolyte interphase layer in V2O5 Li-ion battery cathodes: time and potential effects. J Electrochem Soc. https://doi.org/10.1149/1945-7111/abf17b
Xiao H, Li B, Zhao M (2021) Electrosynthesized CuO x/graphene by a four-electrode electrolysis system for the oxygen reduction reaction to hydrogen peroxide. Chem Commun 57:4118–4121. https://doi.org/10.1039/d1cc00386k
Zhang S, Xie Y, Yang M et al (2021) A defect-rich ultrathin MoS2/rGO nanosheet electrocatalyst for the oxygen reduction reaction. RSC Adv 11:24508–24514. https://doi.org/10.1039/d1ra03552e
Prabhakar Vattikuti SV, Nagajyothi PC, Devarayapalli KC et al (2020) Hybrid Ag/MoS2 nanosheets for efficient electrocatalytic oxygen reduction. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2020.146751
Chen S, Luo T, Li X et al (2022) Identification of the highly active Co–N4 coordination motif for selective oxygen reduction. J Am Chem Soc. https://doi.org/10.1021/jacs.2c01194
Dianhydride P, Applications LB (2018) Glycination: a simple strategy to enhance the cycling performance of perylene dianhydride for secondary Li–Ion battery applications. ChemistrySelect https://doi.org/10.1002/slct.201801588
Acknowledgements
The SERB, New Delhi is acknowledged by the authors for funding the lab infrastructure under the SRG project (Ref: SRG/2019/001090).The authors thank Prof. Kanupriya Sachdev, Dr. Debasish Sarkar and Dr. Ritu Bala for their meaningful advice. The characterizations were done by Material Research Centre (MRC), MNIT Jaipur. The authors also acknowledge MRC.
Author information
Authors and Affiliations
Contributions
Conceptualization, Methodology, experimental, and writing the original draft was done by MS. Conceptualization, validation, review, supervision, and editing of the final manuscript were done by RD. VK and KR reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest reported by the authors that could be perceived as having influenced the work presented in this publication, either through financial means or in any other way.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shrivastav, M., Kumar, V., Rana, K. et al. Oxygen reduction reaction kinetics of 2H-MoS2 and mixed-phase 1T/2H-MoS2 as a metal-free cathodic catalyst for PEM fuel cells. J Appl Electrochem (2024). https://doi.org/10.1007/s10800-023-02053-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10800-023-02053-0