Abstract
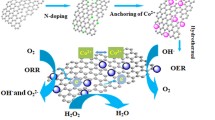
In this paper, we present a study on the synthesis and electrochemical characterization of nitrogen-doped reduced graphene aerogel (NrGA) prepared through the hydrothermal reduction of graphene oxide (GO) suspension with urea as a nitrogen dopant source. Five samples (rGA, NrGA08, NrGA16, NrGA32, NrGA48) with varying concentrations of urea were prepared to investigate the effect of urea concentration on electrochemical performance. All samples were characterized using XRD, FT-IR, Raman, BET, FESEM, and XPS. The specific surface area of the samples ranged from 110 to 344 m2/g, with the highest value observed for NrGA48. Raman spectroscopy showed the generation of disorder in the structure with the insertion of nitrogen atoms. The electrochemical performance of the sample has been investigated through linear sweep voltammetry, cyclic voltammetry, chronoamperometry, and RDE. The NrGA32 sample exhibited superior electrochemical performance compared to the other samples, and was therefore chosen as the optimized N-doped sample to investigate the effect of cobalt phthalocyanine (CoPC) as a redox mediator. The addition of CoPC significantly improved the electrochemical properties of NrGA32, increasing the electron transfer number from 3.5 to 3.85, enhancing the oxygen reduction current, and shifting the onset potential from 0.480 to 0.700 V vs. SHE.
Graphical abstract












Similar content being viewed by others
References
Gómez-Marín A, Feliu J (2018) Oxygen reduction on platinum single crystal electrodes. Encyclopedia Interf Chem Surf Sci Electrochem. https://doi.org/10.1021/acscatal.8b03351
Liang Y et al (2011) Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat Mater 10(10):780–786
Si F et al (2014) Electrochemical oxygen reduction reaction rotating electrode methods and oxygen reduction electrocatalysts. Elsevier, Amsterdam, pp 133–170
Ge X et al (2015) Oxygen reduction in alkaline media: from mechanisms to recent advances of catalysts. ACS Catal 5(8):4643–4667
Gong K et al (2009) Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 323(5915):760–764
Dai L et al (2012) Carbon nanomaterials for advanced energy conversion and storage. small 8(8):1130–1166
Mungse HP et al (2014) Hydrothermal deoxygenation of graphene oxide in sub-and supercritical water. RSC Adv 4(43):22589–22595
Bagri A et al (2010) Structural evolution during the reduction of chemically derived graphene oxide. Nat Chem 2(7):581–587
Tao Y et al (2013) Towards ultrahigh volumetric capacitance: graphene derived highly dense but porous carbons for supercapacitors. Sci Rep 3(1):1–8
Liu J et al (2014) A three-dimensional graphene skeleton as a fast electron and ion transport network for electrochemical applications. J Mater Chem A 2(9):3031–3037
Zhou Y et al (2009) Hydrothermal dehydration for the “green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties. Chem Mater 21(13):2950–2956
Chamoli P, Das MK, Kar KK (2017) Temperature dependence green reduction of graphene oxide by urea. Adv Mater Lett 8(3):217–222
Hu H et al (2013) Ultralight and highly compressible graphene aerogels. Adv Mater 25(15):2219–2223
Sheng K-x et al (2011) High-performance self-assembled graphene hydrogels prepared by chemical reduction of graphene oxide. New Carbon Mater 26(1):9–15
Paraknowitsch JP, Thomas A (2013) Doping carbons beyond nitrogen: an overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ Sci 6(10):2839–2855
Yuan B et al (2016) Boron/phosphorus doping for retarding the oxidation of reduced graphene oxide. Carbon 101:152–158
Terrones M et al (2002) N-doping and coalescence of carbon nanotubes: synthesis and electronic properties. Appl Phys A 74(3):355–361
Zhang G, Duan W, Gu B (2002) Effect of substitutional atoms in the tip on field-emission properties of capped carbon nanotubes. Appl Phys Lett 80(14):2589–2591
Lai L et al (2012) Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ Sci 5(7):7936–7942
Chung JS, Kim EJ, Hur SH (2014) The molecular level control of three-dimensional graphene oxide hydrogel structure by using various diamines. Chem Eng J 246:64–70
Hagel P et al (1971) Cyanate formation in solutions of urea: I. Calculation of cyanate concentrations at different temperature and pH. Biochimica Biophysica Acta Protein Structure 243(3):366–373
Shaw WH, Bordeaux JJ (1955) The decomposition of urea in aqueous media. J Am Chem Soc 77(18):4729–4733
Bull HB et al (1964) The pH of urea solutions. Arch Biochem Biophys 104(2):297–304
Sun L et al (2012) Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage. RSC Adv 2(10):4498–4506
Zhang Y, Su K, Li Z (2018) Graphene oxide composite membranes cross-linked with urea for enhanced desalting properties. J Membr Sci 563:718–725
Vollhardt KPC, Schore NE (2003) Organic chemistry: structure and function. Macmillan
Li J et al (2016) Structural and mechanistic basis for the high activity of Fe–N–C catalysts toward oxygen reduction. Energy Environ Sci 9(7):2418–2432
Dodelet J-P (2006) Oxygen reduction in PEM fuel cell conditions: heat-treated non-precious metal-N 4 macrocycles and beyond N4-macrocyclic metal complexes Springer, Berlin, pp 83–147
Zagal JH, Koper MT (2016) Reactivity descriptors for the activity of molecular MN4 catalysts for the oxygen reduction reaction. Angew Chem Int Ed 55(47):14510–14521
Zagal JH et al (2012) Carbon nanotubes and metalloporphyrins and metallophthalocyanines-based materials for electroanalysis. J Porphyr Phthalocyanines 16(07n08):713–740
Shojaeenezhad SS, Farbod M, Kazeminezhad I (2017) Effects of initial graphite particle size and shape on oxidation time in graphene oxide prepared by Hummers’ method. J Science: Adv Mater Devices 2(4):470–475
Royer D (1961) Evidence for the existence of the permanganyl ion in sulphuric acid solutions of potassium permanganate. J Inorg Nucl Chem 17(1–2):159–167
Kang JH et al (2016) Hidden second oxidation step of Hummers method. Chem Mater 28(3):756–764
Guo H-L et al (2013) Synthesis and characterization of nitrogen-doped graphene hydrogels by hydrothermal route with urea as reducing-doping agents. J Mater Chem A 1(6):2248–2255
Dimiev AM, Tour JM (2014) Mechanism of graphene oxide formation. ACS Nano 8(3):3060–3068
Deng D et al (2011) Toward N-doped graphene via solvothermal synthesis. Chem Mater 23(5):1188–1193
Pavia DL et al (2014) Introduction to spectroscopy. Cengage Learning
Zheng X et al (2017) Hydrothermal reduction of graphene oxide; effect on surface-enhanced Raman scattering. J Raman Spectrosc 48(1):97–103
Hu X et al (2013) Effects of particle size and pH value on the hydrophilicity of graphene oxide. Appl Surf Sci 273:118–121
Thommes M et al (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87(9–10):1051–1069
Vasiliev VP et al (2022) A facile synthesis of Noble-Metal-Free Catalyst based on Nitrogen Doped Graphene Oxide for Oxygen reduction reaction. Materials 15(3):821
Liu J et al (2016) Strain-induced electrostatic enhancements of BiFeO 3 nanowire loops. Phys Chem Chem Phys 18(33):22772–22777
Ahadi K, Mahdavi S-M, Nemati A (2013) Effect of chemical substitution on the morphology and optical properties of Bi1 – xCaxFeO3 films grown by pulsed-laser deposition. J Mater Sci: Mater Electron 24(1):248–252
Tuinstra F, Koenig JL (1970) Raman spectrum of graphite. J Chem Phys 53(3):1126–1130
Bie C et al (2021) Design, fabrication, and mechanism of nitrogen-doped graphene‐based photocatalyst. Adv Mater 33(9):2003521
Wei D et al (2009) Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett 9(5):1752–1758
Xu H, Ma L, Jin Z (2018) Nitrogen-doped graphene: synthesis, characterizations and energy applications. J energy Chem 27(1):146–160
Akhavan O (2010) The effect of heat treatment on formation of graphene thin films from graphene oxide nanosheets. Carbon 48(2):509–519
Zhao J, Liu L, Li F (2015) Graphene oxide: physics and applications, vol 1. Springer
Hwang JO et al (2012) Workfunction-tunable, N-doped reduced graphene transparent electrodes for high-performance polymer light-emitting diodes. ACS Nano 6(1):159–167
Ouyang Z et al (2019) Preparation and specific capacitance properties of sulfur, nitrogen co-doped graphene quantum dots. Nanoscale Res Lett 14(1):1–9
Ahadi K, Cadien K (2021) Hf1 – xZrxO2 and HfO2/ZrO2 gate dielectrics with extremely low density of interfacial defects using low temperature atomic layer deposition on GaN and InP. J Vacuum Sci Technol A: Vacuum Surf Films 39(3):032407
Jin Z et al (2011) Large-scale growth and characterizations of nitrogen-doped monolayer graphene sheets. ACS Nano 5(5):4112–4117
Kianinia M, Ahadi K, Nemati A (2011) Investigation of dark and light conductivities in calcium doped bismuth ferrite thin films. Mater Lett 65(19–20):3086–3088
Al-Tawhid AH et al (2022) Oxygen Vacancy-Induced Anomalous Hall Effect in a nominally non-magnetic oxide. J Electron Mater 51(12):7073–7077
Al-Tawhid AH et al (2022) Superconductivity and weak anti-localization at KTaO3 (111) interfaces. J Electron Mater. https://doi.org/10.1007/s11664-022-09844-9
Schwaigert T et al (2023) Molecular beam epitaxy of KTaO3. J Vacuum Sci Technol A 41(2):022703
Arnault EG et al (2023) Anisotropic superconductivity at KTaO3 (111) interfaces. Sci Adv 9(7):eadf1414
Antić Ž et al (2017) Transparent and highly luminescent dysprosium-doped GdVO4 thin films fabricated by pulsed laser deposition. Thin Solid Films 638:332–337
Al-Tawhid AH, Kumah DP, Ahadi K (2021) Two-dimensional electron systems and interfacial coupling in LaCrO3/KTaO3 heterostructures. Appl Phys Lett 118(19):192905
Wang G et al (2008) Density functional theory study of the adsorption of oxygen molecule on iron phthalocyanine and cobalt phthalocyanine. Mol Simul 34(10–15):1051–1056
Hu H et al (2016) Metal–organic-framework-engaged formation of Co nanoparticle-embedded carbon@ Co 9 S 8 double-shelled nanocages for efficient oxygen reduction. Energy Environ Sci 9(1):107–111
Fernandes DM et al (2019) Towards efficient oxygen reduction reaction electrocatalysts through graphene doping. Electrochim Acta 319:72–81
Jahan M, Bao Q, Loh KP (2012) Electrocatalytically active graphene–porphyrin MOF composite for oxygen reduction reaction. J Am Chem Soc 134(15):6707–6713
Xue Q et al (2018) 3D nitrogen-doped graphene aerogels as efficient electrocatalyst for the oxygen reduction reaction. Carbon 139:137–144
Wang Q, Hu W, Huang Y (2017) Nitrogen doped graphene anchored cobalt oxides efficiently bi-functionally catalyze both oxygen reduction reaction and oxygen revolution reaction. Int J Hydrog Energy 42(9):5899–5907
Tian GL et al (2014) Nitrogen-doped graphene/carbon nanotube hybrids: in situ formation on bifunctional catalysts and their superior electrocatalytic activity for oxygen evolution/reduction reaction. Small 10(11):2251–2259
Yu D et al (2017) Nitrogen-doped graphene aerogels-supported cobaltosic oxide nanocrystals as high-performance bi-functional electrocatalysts for oxygen reduction and evolution reactions. J Electroanal Chem 787:46–54
Liu Y et al (2015) Nitrogen-doped graphene aerogel-supported spinel CoMn2O4 nanoparticles as an efficient catalyst for oxygen reduction reaction. J Power Sources 299:492–500
Shao Y et al (2019) Progress in nonmetal-doped graphene electrocatalysts for the oxygen reduction reaction. Chemsuschem 12(10):2133–2146
Zhang C et al (2012) Iron phthalocyanine and nitrogen-doped graphene composite as a novel non-precious catalyst for the oxygen reduction reaction. Nanoscale 4(23):7326–7329
Gautam RK et al (2016) Nitrogen doped graphene supported α-MnO 2 nanorods for efficient ORR in a microbial fuel cell. RSC Adv 6(111):110091–110101
Wu Z-S et al (2012) 3D nitrogen-doped graphene aerogel-supported Fe3O4 nanoparticles as efficient electrocatalysts for the oxygen reduction reaction. J Am Chem Soc 134(22):9082–9085
Author information
Authors and Affiliations
Contributions
SA, and YG were the main authors of the manuscript and creators of the figures. All experiments were done by SA, and YG. The research conducted under supervision of AD.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahadi, S., Ghorbani, Y. & Dolati, A. Electrochemical characterization of 3D N-rGO with cobalt phthalocyanine as redox mediator toward oxygen reduction reaction. J Appl Electrochem 53, 2197–2212 (2023). https://doi.org/10.1007/s10800-023-01918-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-01918-8