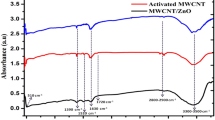
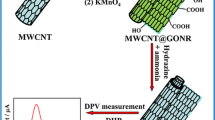
Abstract
A series of 2-(4-(1-isonicotinoyl-5-aryl-4,5-dihydro-1H-pyrazol-3-yl)phenoxy)-N-arylacetamides has been synthesized in appreciable yields. The quantitative determination and redox behavior of the synthesized compounds were estimated by cyclic voltammetry (CV). Further, it was observed that nanocomposites have come to be the superior modifying materials for electrochemical sensing. Herein, a highly sophisticated and sensitive zinc oxide (ZnO)-multi walled carbon nanotubes (MWCNTs) composite film modified glassy carbon electrode (GCE) (ZnO-MWCNTs/GCE) was fabricated for its use as the working electrode. Prior to the fabrication, the synthesized ZnO material was characterized by SEM to confirm the successful synthesis. After the fabrication of the ZnO-MWCNTs/GCE sensor, it together with its corresponding forms i.e., ZnO/GCE and MWCNTs/GCE including bare GCE was characterized by voltammetry. The increase in effective surface area of modified GCE from that of unembellished GCE of 0.0314 cm2 up to 0.081 cm2 resulted in better electrocatalytic activity in terms of responses of the compounds under investigation. The composite film modified GCE resulted in an excellent sensor for the reported compounds in terms of low potential detection, a low detection limit, fast and clear response. Moreover, the effect of pH, varying scan rates, solvents, electrolytes, concentration, and substituents (in terms of the Hammett equation) have also been analyzed on the CV responses of the modified GCE. The best results were obtained for a pH value corresponding to 5.83 with TEAP as the surfactant at the scan rate of 0.1 V/s. The incorporation of ZnO nanoparticles with multi walled carbon nanotubes (MWCNTs) greatly increases the voltammetric peak current and electrochemical reactivity of the compounds. The electrochemical sensor shows sensitivity par excellence and exhibits a wide detection range varying from 1.0 × 10–4 to 3.0 × 10–4 M. Moreover, the detection limit is 1.25 × 10−6 M with a correlation coefficient (r2) of 0.994. The studies delineated the diffusion-controlled and irreversible behavior of electrocatalytic reaction for all the compounds under investigation.
Graphic Abstract













Similar content being viewed by others
References
Kissinger PT, Heineman WR (1996) Laboratory techniques in electroanalytical chemistry, 2nd edn. Dekker, New York
Vittal R, Gomathi H, Kim KJ (2006) Beneficial role of surfactants in electrochemistry and in the modification of electrodes. Adv Colloid Interface Sci 119:55–68. https://doi.org/10.1016/j.cis.2005.09.004
Wang J (2000) Analytical electrochemistry, 2nd edn. Wiley, New York
Abdullah HM, Rania RZ, Esam AG, Mahmoud NAEl-Hady (2022) Bivalent transition metal complexes of pyridine-2,6-dicarbohydrazide: Structural characterization, cyclic voltammetry and biological studies. J Mol Struct 1269:133852. https://doi.org/10.1016/j.molstruc.2022.133852.
Kumar PS, Sreeja BS, Kumar KK, Padmalaya G (2022) Investigation of Nafion coated GO-ZnO nanocomposite behaviour for sulfamethoxazole detection using cyclic voltammetry. Food Chem Toxicol 167:113311. https://doi.org/10.1016/j.fct.2022.113311.
Gupta VK, Jain R, Radhapyari K, Jadon N, Agarwal S (2011) Voltammetric techniques for the assay of pharmaceuticals—a review. Anal Biochem 408:179–196. https://doi.org/10.1016/j.ab.2010.09.027
Ghassab N, Soleymanpour A, Shafaatian B (2022) Development of an ultrasensitive chemically modified carbon paste electrode for selective determination trace amount of sulfate ion. Measurement 205:112231. https://doi.org/10.1016/j.measurement.2022.112231.
Zare HR, Nasirizadeh N (2010) Simultaneous determination of ascorbic acid, adrenaline and uric acid at a hematoxylin multi-wall carbon nanotube modified glassy carbon electrode. Sens Actuators B 143:666–672. https://doi.org/10.1016/j.snb.2009.10.030
Carmichael AJ, Seddon KR (2000) Polarity study of some 1-alkyl-3-methylimidazolium ambient-temperature ionic liquids with the solvatochromic dye, Nile Red. J Phys Org Chem 13:591–595
Shrivastava S, Jadon N, Jain R (2016) Next-generation polymer nanocomposite-based electrochemical sensors and biosensors: a review. Trends Analt Chem 82:55–67. https://doi.org/10.1016/j.trac.2016.04.005
Zhang C, Wang G, Liu M, Feng Y, Zhang Z, Fang B (2010) A hydroxylamine electrochemical sensor based on electrodeposition of porous ZnO nanofilms onto carbon nanotubes films modified electrode. Electrochim Acta 55:2835–2840. https://doi.org/10.1016/j.electacta.2009.12.068
Maheshwaran S, Tamilalagan E, Chen S-M, Akilarasan M, Huang Y-F, AlMasoud N, Abualnaja KM, Ouladsmne M (2021) Rationally designed f-MWCNT-coated bismuth molybdate (f-MWCNT@BMO) nanocomposites for the voltammetric detection of biomolecule dopamine in biological samples. Microchim Acta 188:315. https://doi.org/10.1007/s00604-021-04978-9
Jain R, Sharma RK (2012) Novel bismuth/multi-walled carbon nanotubes-based electrochemical sensor for the determination of neuroprotective drug cilostazol. J Appl Electrochem 42:341–348. https://doi.org/10.1007/s10800-012-0402-8
Periasamy AP, Yang S, Chen SM (2011) Preparation and characterization of bismuth oxide nanoparticles-multiwalled carbon nanotube composite for the development of horseradish peroxidase based H2O2 biosensor. Talanta 87:15–23. https://doi.org/10.1016/j.talanta.2011.09.021
Jiang LC, Zhang WD (2009) Electrodeposition of TiO2 nanoparticles on multiwalled carbon nanotube arrays for hydrogen peroxide sensing. Electroanalysis 21:988–993. https://doi.org/10.1002/elan.200804502
Gupta VK, Jain R, Agarwal S, Mishra R, Dwivedi A (2011) Electrochemical determination of antihypertensive drug irbesartan in pharmaceuticals. Anal Biochem 410:266–271. https://doi.org/10.1016/j.ab.2010.11.024
Arun V, Prabhu S, Priyadharsan A, Maadeswaran P, Sohila S, Ramesh R, Kumar AS (2021) Facile, low cost synthesis of cauliflower-shaped ZnO with MWCNT/rGO nanocomposites and their photocatalytic activity. J Mater Sci: Mater Electron 32:15763–15777. https://doi.org/10.1007/s10854-021-06129-5
Prabhu K, Malode SJ, Shetti NP, Kulkarni RM (2022) Analysis of herbicide and its applications through a sensitive electrochemical technique based on MWCNTs/ZnO/CPE fabricated sensor. Chemosphere 287: 132086. https://doi.org/10.1016/j.chemosphere.2021.132086.
Westmoreland PG, Day RA, Underwood AL (1972) Electrochemistry of substances solubilized in micelles. Polarography of azobenzene in aqueous surfactant solutions. Anal Chem 44:737–740. https://doi.org/10.1021/ac60312a060
Ziyatdinova G, Giniyatova E, Budnikov H (2010) Cyclic voltammetry of retinol in surfactant media and its application for the analysis of real samples. Electroanalysis 22:2708–2713. https://doi.org/10.1002/elan.201000358
Jain R, Dwivedi A, Mishra R (2009) Adsorptive stripping voltammetric behavior of nortriptyline hydrochloride and its determination in surfactant media. Langmuir 25:10364–10369. https://doi.org/10.1021/la900927j
Levent Y, Yardim Z, Senturk, (2009) Voltammetric behavior of nicotine at pencil graphite electrode and its enhancement determination in the presence of anionic surfactant. Electrochim Acta 55:190–195. https://doi.org/10.1016/j.electacta.2009.08.035
Elgrishi NM, Rountree KJ, McCarthy BD, Rountree ES, Eisenhart TT, Dempsey JL (2018) A practical beginner’s guide to cyclic voltammetry. J Chem Educ 95:197–206. https://doi.org/10.1021/acs.jchemed.7b00361
Ma L, Xie C, Ma Y, Liu J, Xiang M, Ye X, Zheng H, Chen Z, Xu Q, Chen T, Chen J, Yang J, Qiu N, Wang G, Liang X, Peng A, Yang S, Wei Y, Chen L (2011) Synthesis and biological evaluation of novel 5-benzylidenethiazolidine-2,4-dione derivatives for the treatment of inflammatory diseases. J Med Chem 54:2060–2068. https://doi.org/10.1021/jm1011534
Reen GK, Ahuja M, Kumar A, Patidar R, Sharma P (2017) ZnO nanoparticle-catalyzed multicomponent reaction for the synthesis of 1,4-diaryl dihydropyridines. Org Prep Proced Int 49:273–286. https://doi.org/10.1080/00304948.2017.1320927
Jain R, Sinha A, Kumari N, Khan AL (2016) A polyaniline/graphene oxide nanocomposite as a voltammetric sensor for electroanalytical detection of clonazepam. Anal Methods 8:3034–3045. https://doi.org/10.1039/c6ay00424e
Murugan N, Kumar THV, Devi R, Sundramoorthy AK (2019) A flower-structured MoS2-decorated f-MWCNTs/ZnO hybrid nanocomposite-modified sensor for the selective electrochemical detection of vitamin C. New J Chem 43:15105–15114. https://doi.org/10.1039/c9nj02993a
Britton HTS (1956) Hydrogen ions, vol I. Van Nostrand Co., New York
Bard AJ, Faulkner LR (2010) Electrochemical methods: fundamentals and applications. Wiley, New York
Abou-Elenien GM, Ismail NA, El-Maghraby AA, Al-abdallah GM (2001) Electrochemical studies on some pyrazole, oxadiazole, and thiadiazole derivatives. Electroanalysis 13:1022–1029. https://doi.org/10.1002/1521-4109(200108)13:12%3C1022::aid-elan1022%3E3.0.co;2-x
Kumar KR, Prasad ARG, Srilalitha V, Swamy GN, Ravindranath LK (2012) Synthesis and electrochemical investigations on certain pyrazolin-5-ones. Scientica Iranica 19:605–618. https://doi.org/10.1016/j.scient.2012.02.025
Beloglazkina EK, Korablina DD, Vorozhtsov NI, Sviridova LA, Moiseeva AA, Skvortsov DA, Rybakov VB, Majouga AG, Zyk NV (2019) Synthesis of 3-(pyridine-2-yl)-4,5-dihydro-1H-pyrazole-1-thiocarboxamides and their copper(II) complexes. Arab J Chem 12:1050–1060. https://doi.org/10.1016/j.arabjc.2017.01.005
Author information
Authors and Affiliations
Contributions
G.K.R. wrote the manuscript, and A.K. and P.S. helped with experimental work and result analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Reen, G.K., Kumar, A. & Sharma, P. Fabrication and optimization of ZnO-multiwalled carbon nanotubes modified glassy carbon electrode for the detection of 2-(4-(1-isonicotinoyl-5-aryl-4,5-dihydro-1H-pyrazol-3-yl)phenoxy)-N-arylacetamides. J Appl Electrochem 53, 1739–1753 (2023). https://doi.org/10.1007/s10800-023-01889-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-01889-w