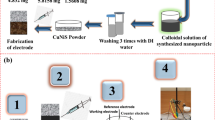
Abstract
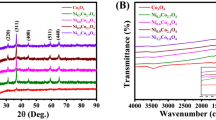
Nickel-doped CuO/Cu/Cu2O nanocomposites were prepared by simple precipitation method. Nickel was doped at three different concentrations of 2 mM (Ni-Cu2O/CuO/Cu (NCO-1)), 4 mM (Ni-CuO/Cu/Cu2O (NCO-2)), and 6 mM (Ni-Cu2O/Cu (NCO-3) and was used as an electrode material for glucose sensing and energy storage applications. The X-ray diffraction spectroscopy (XRD) confirms the formation of different phases of CuO, Cu2O, and Cu in the NCO nanocomposites. Among the NCO samples, the NCO-2 nanocomposite showed two different surface morphologies of spherical-shaped nanoparticles with an average size of 35.97 nm and nanoflakes with an average length of 64.75 nm and a width of 25.21 nm. The X-ray photoelectron spectroscopy (XPS) analysis of NCO-2 confirms the presence of mixed oxidation states of Cu2+, Cu1+, Cu0, and Ni2+. The electrochemical performance of NCO-2 nanocomposite is high compared to the other NCO composite which shows glucose detection sensitivity of 208.5 µA mM−1 cm−2 and Limit of detection (LOD) of above 0.7 µM for a linear range of 0.01 to 8 mM. Also, in supercapacitor application, the NCO-2 electrode material exhibits a high specific capacitance of 338.98 F g−1 at the current density of 1 A g−1 in 1-M KOH electrolyte compared to the other NCO composites. The aqueous asymmetric supercapacitor (ASC) device of cell configuration AC//Ni-doped CuO/Cu/Cu2O was fabricated which showed an energy density of 10.03 Wh kg−1 and power density of 874.58 W kg−1 at current density 1 A g−1. The device showed excellent cyclic stability with a capacitance retention of 87.61% and good coulombic efficiency of 94.28% after 5000 cycles.
Graphical abstract


















Similar content being viewed by others
References
Prabhakaran A, Nayak P (2020) Surface engineering of laser-scribed graphene sensor enables non-enzymatic glucose detection in human body fluids. ACS Appl Nano Mater 3:391–398
Bag S, Baksi A, Nandam SH, Wang D, Ye X, Ghosh J, Pradeep T, Hahn H (2020) Nonenzymatic glucose sensing using Ni60Nb40 nanoglass. ACS Nano 14:5543–5552
Karikalan N, Velmurugan M, Chen SM, Karuppiah C (2016) Modern approach to the synthesis of Ni(OH)2 decorated sulfur doped carbon nanoparticles for the nonenzymatic glucose sensor. ACS Appl Mater Interfaces 8:22545–22553
Chen T, Liu D, Lu W, Wang K, Du G, Asiri AM, Sun X (2016) Three dimensional Ni2P nanoarray: an efficient catalyst electrode for sensitive and selective nonenzymatic glucose sensing with high specificity. Anal Chem 88:7885–7889
Wei M, Qiao Y, Zhao H, Liang J, Li T, Luo Y, Lu S, Shi X, Lu W, Sun X (2020) Electrochemical non-enzymatic glucose sensors: recent progress and perspectives. Chem Commun 56:14553
Qiao Y, Liu Q, Lu S, Chen G, Gao S, Lu W, Sun X (2020) High-performance non-enzymatic glucose detection: using conductive Ni-MOF as an electrocatalyst. J Mater Chem B 8:5411–5415
Zhang H, Yu Y, Shen X, Hu XA (2020) Cu2O/Cu/carbon cloth as a binder-free electrode for non-enzymatic glucose sensors with high performance. New J Chem 44:1993–2000
Gowthaman NSK, Arul P, Lim HN, John SA (2020) Negative potential-induced growth of surfactant-free CuO nanostructures on an al-c substrate: a dual in-line sensor for biomarkers of diabetes and oxidative stress. ACS Sustain Chem Eng 8:2640–2651
Ahmad R, Tripathy N, Hahn Y, Umar A, Ibrahim A, Kim S (2015) A robust enzymeless glucose sensor based on CuO nanoseed modified electrodes. Dalt Trans 44:12488–12492
Saraf M, Natarajan K, Mobin SM (2016) Non-enzymatic amperometric sensing of glucose by employing sucrose templated microspheres of copper oxide (CuO). Dalt Trans 45:5833–5840
Dong C, Wang Y, Xu J, Cheng G, Yang W, Kou T, Zhang Z, Ding Y (2014) 3D binder-free Cu2O@Cu nanoneedle arrays for high-performance asymmetric supercapacitors. J Mater Chem A 2:18229–18235
Lin LY, Karakocak BB, Kavadiya S, Soundappan T, Biswas P (2017) A highly sensitive non-enzymatic glucose sensor based on Cu/Cu2O/CuO ternary composite hollow spheres prepared in a furnace aerosol reactor. Sens Actuators B. https://doi.org/10.1016/j.snb.2017.12.035
Huang BR, Wang MJ, Kathiravan D, Kurniawan A, Zhang HH, Yang WL (2018) Interfacial effect of oxygen-doped nanodiamond on CuO and micropyramidal silicon heterostructures for efficient nonenzymatic glucose sensor. ACS Appl Bio Mater 1:1579–1586
Amin BG, Masud J, Nath M (2019) A non-enzymatic glucose sensor based on a CoNi2Se4/rGO nanocomposite with ultrahigh sensitivity at low working potential. J Mater Chem B 7:2338–2348
Nguyen TNH, Jin X, Nolan JK, Xu J, Le KVH, Lam S, Wang Y, Alam MA, Lee H (2020) Printable nonenzymatic glucose biosensors using carbon nanotube-PtNP nanocomposites modified with AuRu for improved selectivity. ACS Biomater Sci Eng 6:5315–5325
Tomanin PP, Cherepanov PV, Besford QA, Christofferson AJ, Amodio A, McConville CF, Yarovsky I, Caruso F, Cavalieri F (2018) Cobalt phosphate nanostructures for non-enzymatic glucose sensing at physiological pH. ACS Appl Mater Interfaces 10:42786–42795
Deshmukh MA, Kang BC, Ha TJ (2020) Non-enzymatic electrochemical glucose sensors based on polyaniline/reduced-graphene-oxide nanocomposites functionalized with silver nanoparticles. J Mater Chem C 8:5112–5123
Soomro RA, Ibupoto ZH, Sirajuddin AMI, Willander M (2015) Electrochemical sensing of glucose based on novel hedgehog-like NiO nanostructures. Sens Actuators B Chem 209:966–974
Li Y, Chang S, Liu X, Huang J, Yin J, Wang G, Cao D (2012) Nanostructured CuO directly grown on copper foam and their supercapacitance performance. Electrochim Acta 85:393–398
Arun L, Karthikeyan C, Philip D, Sasikumar M, Elanthamilan E, Merlin JP, Unni C (2018) Effect of Ni2+ doping on chemocatalytic and supercapacitor performance of biosynthesized nanostructured CuO. J Mater Sci Mater Electron 29:21180–21193
Bouazizi N, Vieillard J, Thebault P, Desirac F, Clamens T, Bargougui R, Couvrat N, Thoumire O, Brun N, Ladam G, Morin S, Mofaddel N, Lesouhaitier O, Azzouz A, Derf FL (2018) Silver nanoparticle embedded copper oxide as an efficient core-shell for the catalytic reduction of 4-nitrophenol and antibacterial activity improvement. Dalt Trans 47:9143–9155
Yin W, Yang J, Zhao K, Cui A, Zhou J, Tian W, Li W, Hu Z, Chu J (2020) High responsivity and external quantum efficiency photodetectors based on solution-processed Ni-doped CuO films. ACS Appl Mater Interfaces 12:11797–11805
Chen YC, Chen YJ, Dong PH, Hsu YK (2020) Benchmarked photoelectrochemical water splitting by nickel-doped n-type cuprous oxide. ACS Appl Energy Mater 3:1373–1380
Wang W, Jiang Y, Hu Y, Liu Y, Li J, Chen S (2020) Top-open hollow nanocubes of Ni-doped Cu oxides on Ni foam: scalable oxygen evolution electrode via galvanic displacement and face-selective etching. ACS Appl Mater Interfaces 12:11600–11606
Ming LQ, Bi ZD, Yamamoto Y, Ichino R, Okido M (2012) Preparation of Cu nanoparticles with NaBH4 by aqueous reduction method. Trans Nonferrous Met Soc China 22:117–123
Yang J, Yin W, Zhou B, Cui A, Xu L, Zhang D, Li W, Hu Z, Chu J (2019) Composition dependence of optical properties and band structures in p-type Ni-doped CuO films: spectroscopic experiment and first-principles calculation. J Phys Chem C 123:27165–27171
Nithya S, Sharan R, Roy M, Kim HH, Ishihara T, Dutta A (2019) Ni doping in CuO: a highly sensitive electrode for sensing ammonia in ppm level using lanthanum gallate based electrolyte. Mater Res Bull 118:110478
Ghodselahi T, Vesaghi MA, Shafiekhani A, Baghizadeh A, Lameii M (2008) XPS study of the Cu@Cu2O core-shell nanoparticles. Appl Surf Sci 255:2730–2734
Zhang F, Xiao X, Wang J, Huang S, Zhang H, Zhang W, Guo X, Zhang D, Wang M (2019) Facile synthesis of uniform CuO/Cu2O composite hollow microspheres for a non-enzymatic glucose sensor. Mater Res Express 6:115049
Liu L, Qi W, Gao X, Wang C, Wang G (2018) Synergistic effect of metal ion additives on graphitic carbon nitride nanosheet-templated electrodeposition of Cu@CuO for enzyme-free glucose detection. J Alloys Compd 745:155–163
Naghian E, Khosrowshahi EM, Sohouli E, Ahmadi F, Nasrabadi MR, Safarifard V (2020) A new electrochemical sensor for the detection of fentanyl lethal drug by a screen-printed carbon electrode modified with the open-ended channels of Zn(ii)-MOF. New J Chem 44:9271–9277
Rajpurohit AS, Punde NS, Srivastava AK (2019) An electrochemical sensor with a copper oxide/gold nanoparticle-modified electrode for the simultaneous detection of the potential diabetic biomarkers methylglyoxal and its detoxification enzyme glyoxalase. New J Chem 43:16572–16582
Wu KL, Cai YM, Jiang BB, Cheong WC, Wei XW, Wanga W, Yua N (2017) Cu@Ni core-shell nanoparticles/reduced graphene oxide nanocomposites for nonenzymatic glucose sensor. RSC Adv 7:21128–21135
Song J, Xu L, Zhou C, Xing R, Dai Q, Liu D, Song H (2013) Synthesis of graphene oxide based CuO nanoparticles composite electrode for highly enhanced nonenzymatic glucose detection. ACS Appl Mater Interfaces 5:12928–12934
Sivakumar M, Madhu R, Chen SM, Veeramani V, Manikandan A, Hung WH, Miyamoto N, Chueh YL (2016) Low-temperature chemical synthesis of CoWO4 nanospheres for sensitive nonenzymatic glucose sensor. J Phys Chem C 120:17024–17028
Patil AS, Patil RT, Logar GM, Fulari VJ (2021) Facile synthesis of CuO nanostructures for non-enzymatic glucose sensor by modified SILAR method. Appl Phys A 127:101
Vediyappan V, Sivakumar M, Chen SM, Lai Q, Madhu R (2021) Nanolayers of carbon protected copper oxide nanocomposite for high performance energy storage and non-enzymatic glucose senor. J Alloys Compd 875:160063
Annalakshmi M, Kumaravel S, Chen TM, Chen SM, Lou BS (2021) 3D flower like NiCo layered double hydroxides; an efficient electrocatalyst for non-enzymatic hydrogen peroxide in live cells and glucose in biofluids. ACS Appl Bio Mater 4:3203–3213
Kuzhandaivel H, Manickam S, Balasingam SK, Franklin MC, Kim HJ, Nallathambi KS (2021) Sulfur and nitrogen-doped graphene quantum dots/PANI nanocomposites for supercapacitors. New J Chem 45:4101–4110
Khatavkar SN, Sartale SD (2020) Superior supercapacitive performance of grass-like CuO thin films deposited by liquid phase deposition. New J Chem 44:6778–6790
Hussain I, Mak T, Zhang K (2021) Boron-doped trimetallic Cu-Ni-Co oxide nanoneedles for supercapacitor application. ACS Appl Nano Mater 4:129–141
Kuzhandaivel H, Selvaraj Y, Franklin MC, Manickam S, Nallathambi KS (2021) Low-temperature-synthesized Mn-doped Bi2Fe4O9 as an efficient electrode material for supercapacitor applications. New J Chem 45:15223–15233
Xu P, Ye K, Du M, Liu J, Cheng K, Yin J, Wang G, Cao D (2015) One-step synthesis of copper compounds on copper foil and their supercapacitive performance. RSC Adv 5:36656–36664
Dubal DP, Gund GS, Lokhande CD, Holze R (2013) CuO cauliflowers for supercapacitor application: novel potentiodynamic deposition. Mat Res Bull 48:923–928
Wang S, Jiang L, Hu J, Wang Q, Zhan S, Lu Y (2020) Dual-functional CuxO/Cu electrodes for supercapacitors and non-enzyamtic glucose sensors fabricated by femtosecond laser enhanced thermal oxidation. J Alloys Compd 815:152105
Shinde AV, Chodankar NR, Lokhande VC, Lokhande AC, Ji T, Kim JH, Lokhande CD (2016) Highly energetic flexible all-solid-state asymmetric supercapacitor with Fe2O3 and CuO thin films. RSC Adv 6:58839–58843
Cao Y, Liang J, Li X, Yue L, Liu Q, Lu S, Asiri AM, Hu J, Luo Y, Sun X (2021) Recent advances in perovskite oxides as electrode materials for supercapacitors. Chem Commun 57:2343
Troschke E, Oschatz M, Ili IK (2021) Schiff-bases for sustainable battery and supercapacitor electrodes. Exploration 3:20210128
Wang T, Wang Y, Lei J, Chen KJ, Wang H (2021) Electrochemically induced surface reconstruction of Ni-Co oxide nanosheet arrays for hybrid supercapacitors. Exploration 3:20210178
Omar H, Abd-Elkader DNM (2013) Synthesis and characterization of new copper based nanocomposite. Int J Electrochem Sci 8:8614–8622
Acknowledgements
The authors Hemalatha Kuzhandaivel and Kiruthika Paramasivam would like to thank the Technical Education and Quality Improvement Program, Phase-III (TEQIP-III), for the financial assistance and facilities to carry out this research work. Also we thank the management and principal of Coimbatore Institute of Technology for their constant encouragement and support. The author Karhick Sivalingam Nallathambi would like to thank MHRD-RUSA 2.0—BCTRC (Bharathiar Cancer Theranostics Research Center) for the financial support.
Funding
This study is supported by the TEQIP—PHASE III, Rashtriya Uchchatar Shiksha Abhiyan (RUSA), 2.0
Author information
Authors and Affiliations
Contributions
HK and KP planned the work and prepared all the samples and wrote the results and discussion part. SM helped the synthesis part and checking the graphs and their results. KSN correcting the results and discussion part.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuzhandaivel, H., Paramasivam, K., Manickam, S. et al. Nickel-doped CuO/Cu/Cu2O nanocomposite as an efficient electrode for electrochemical non-enzymatic glucose sensor and asymmetric supercapacitor. J Appl Electrochem 53, 1869–1886 (2023). https://doi.org/10.1007/s10800-023-01887-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-01887-y