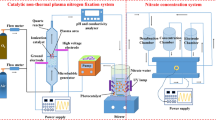
Abstract
The plasma electrolysis method using N2 and O2 injection is an effective and environmentally friendly solution for nitrogen fixation into nitrate and ammonia. The reaction pathway, the effect of the N2 and O2 gas injection composition are important parameters in understanding the mechanism and effectiveness of these processes. This study aims to determine the formation pathway of nitrate and ammonia by observing the formation and role of reactive species as well as intermediate compounds. Two reaction pathways of NOx and ammonia formation have been observed. The NOx compound formed in the solution was oxidized by ∙OH to NO2, followed by the production of a stable nitrate compound. The ammonium produced from the ammonia pathway was generated from nitrogen reacting with ∙H from H2O. The amount of NH3 formed was lesser compared to the NOx compounds in the liquid and gas phases. This indicates that the NOx pathway is more dominant than that of ammonia. The gas injection test with a ratio of N2/O2 = 79/21 was the most effective for nitrate formation compared to another ratio. The results of the emission intensity measurement test show that the reactive species ∙N, ∙N2*, ∙N2+, ∙OH, and ∙O have a significant role in the nitrate formation through the NOx pathway, while the reactive species ∙N and ∙H lead to the formation of NH3. The highest nitrate product was obtained at a ratio of N2/O2: 79/21 by 1889 mg L−1, while the highest ammonia product reached 31.5 mg L−1 at 100% N2 injection.
Graphic Abstract






Similar content being viewed by others
6. References
Patil B, Wang Q, Hessel V, Lang J (2015) Plasma N2-fixation: 1900–2014. Catal Today 256:49–66
Rouwenhorst KHR, Krzywda PM, Benes NE, Mul G, Lefferts L (2020) 2020 Ammonia production technologies. Techno-Economic Challenges of Green Ammonia as Energy Vector. Elsevier, Amsterdam, pp 41–84
Wang J, Song M, Chen B, Wang L, Zhu R (2017) Effects of pH and H2O2 on ammonia, nitrite, and nitrate transformations during UV254nm irradiation: Implications to nitrogen removal and analysis. Chemosphere 184:1003–1011
Lee B, Winter LR, Lee H, Lim D, Lim H, Elimelech M (2022) Pathways to a Green Ammonia Future. ACS Energy Lett 7(9):3032–3038. https://doi.org/10.1021/acsenergylett.2c01615
Wang Y-Y, Cheng Y-H, Chen K-E, Tsay Y-F (2018) Nitrate transport, signaling, and use efficiency. Annu Rev Plant Biol 69:85–122
Cherkasov N, Ibhadon A, Fitzpatrick P (2015) A review of the existing and alternative methods for greener nitrogen fixation. Chem Eng Process 90:24–33
Hawtof R, Ghosh S, Guarr E, Xu C, Mohan Sankaran R, Renner JN (2019) Catalyst-free, highly selective synthesis of ammonia from nitrogen and water by a plasma electrolytic system. Sci Adv. https://doi.org/10.1126/sciadv.aat5778
Sun J et al (2021) A hybrid plasma electrocatalytic process for sustainable ammonia production. Energy Environ Sci 14(2):865–872. https://doi.org/10.1039/D0EE03769A
Al Zoubi W, Kim MJ, Kim YG, Ko YG (2020) Dual-functional crosslinked polymer-inorganic materials for robust electrochemical performance and antibacterial activity. Chem Eng J 392:123654. https://doi.org/10.1016/j.cej.2019.123654
Al Zoubi W, Kamil MP, Fatimah S, Nashrah N, Ko YG (2020) Recent advances in hybrid organic-inorganic materials with spatial architecture for state-of-the-art applications. Prog Mater Sci 112:100663. https://doi.org/10.1016/j.pmatsci.2020.100663
Wang W, Patil B, Heijkers S, Hessel V, Bogaerts A (2017) Nitrogen fixation by gliding arc plasma: better insight by chemical kinetics modelling. Chemsuschem 10(10):2145–2157
Farawan B, Yusharyahya RD, Gozan M, Saksono N (2021) A novel air plasma electrolysis (APE) with direct air injection in plasma zone to produce nitrate in degradation of organic textile dye. Environ Prog Sustain Energy. https://doi.org/10.1002/ep.13691
Sharma RK et al (2021) Plasma activated electrochemical ammonia synthesis from nitrogen and water. ACS Energy Lett 6(2):313–319. https://doi.org/10.1021/acsenergylett.0c02349
Farisah S, Karamah E, Saksono N (2021) Air plasma electrolysis method for synthesis of liquid nitrate fertilizer with K2HPO4 and K2SO4 electrolytes. Int J Plasma Environ Sci Technol 15(1):e01005
Sakakura T, Takatsuji Y, Morimoto M, Haruyama T (2020) Nitrogen fixation through the plasma/liquid interfacial reaction with controlled conditions of each phase as the reaction locus. Electrochemistry 88(3):190–194
Li S, Medrano JA, Hessel V, Gallucci F (2018) Recent progress of plasma-assisted nitrogen fixation research: a review. Processes 6(12):248
Jiang B et al (2014) Review on electrical discharge plasma technology for wastewater remediation. Chem Eng J 236:348–368. https://doi.org/10.1016/j.cej.2013.09.090
Gao J et al (2008) Degradation of anionic dye eosin by glow discharge electrolysis plasma. Plasma Sci Technol 10:422–427
Liu Y, Sun B, Wang L, Wang D (2012) Characteristics of light emission and radicals formed by contact glow discharge electrolysis of an aqueous solution. Plasma Chem Plasma Process 32(2):359–368
Bruggeman P et al (2016) Plasma–liquid interactions: a review and roadmap. Plasma Sources Sci Technol 25(5):053002
Tsuchida Y, Murakami N, Sakakura T, Takatsuji Y, Haruyama T (2021) Drastically increase in atomic nitrogen production depending on the dielectric constant of beads filled in the discharge space. ACS Omega 6(44):29759–29764
Luvita V, Sugiarto A, Bismo S (2022) Characterization of dielectric barrier discharge reactor with nanobubble application for industrial water treatment and depollution. S Afr J Chem Eng 40:246–257
Sen Gupta SK (2017) Contact glow discharge electrolysis: a novel tool for manifold applications. Plasma Chem Plasma Process 37(4):897–945. https://doi.org/10.1007/s11090-017-9804-z
Sukreni T, Saksono N, Bismo S (2018) Air injection effect on energy consumption and production of hydroxyl radicals at plasma anode. J Environ Sci Technol 12:132–138
Lukes P, Locke BR (2005) Plasmachemical oxidation processes in a hybrid gas–liquid electrical discharge reactor. J Phys D Appl Phys 38(22):4074
Gupta SKS (2015) Contact glow discharge electrolysis: its origin, plasma diagnostics and non-faradaic chemical effects. Plasma Sources Sci Technol 24(6):063001
Yasuoka K, Sato K (2009) Development of repetitive pulsed plasmas in gas bubbles for water treatment. Int J Plasma Environ Sci Technol 3(1):22–27
Burlica R, Kirkpatrick MJ, Locke BR (2006) Formation of reactive species in gliding arc discharges with liquid water. J Electrostat 64(1):35–43
Chen HH, Chen YK, Chang HC (2012) Evaluation of physicochemical properties of plasma treated brown rice. Food Chem 135(1):74–79
Huang L, Li L, Dong W, Liu Y, Hou H (2008) Removal of ammonia by OH radical in aqueous phase. Environ Sci Technol 42(21):8070–8075
Chen H, Yuan D, Wu A, Lin X, Li X (2021) Review of low-temperature plasma nitrogen fixation technology. Waste Disposal Sustain Energy 3(3):201–217
Acknowledgements
The research was partially funded by Publikasi Terindeks International (PUTI) Q2 2022 with contract number NKB-704/UN2.RST/HKP.05.00/2022. The authors declare no competing interest or any conflicts of financial interests
Funding
Publikasi Terindeks International (PUTI),NKB-704/UN2.RST/HKP.05.00/2022,NKB-704/UN2.RST/HKP.05.00/2022
Author information
Authors and Affiliations
Contributions
Nelson Saksono: Conceptualization, Methodology, Writing - Review & Editing, Project administration Harianingsih: Conceptualization, Writing - Original Draft Bening Farawan: Visualization, data curation Veny Luvita: Data curation, Validation Zainal Zakaria: Supervision, Formal analysis
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saksono, N., Harianingsih, Farawan, B. et al. Reaction pathway of nitrate and ammonia formation in the plasma electrolysis process with nitrogen and oxygen gas injection. J Appl Electrochem 53, 1183–1191 (2023). https://doi.org/10.1007/s10800-023-01849-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-01849-4