Abstract
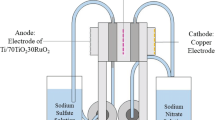
Pesticides, fine chemicals, and many other chemical industries usually produce a large amount of waste solid salt which is detrimental to the environment when treated by burning and rigid landfill. In contrast to traditional disposal strategies, resource utilization of waste salt is beneficial for both the environment and economy. However, the current technique for the resource utilization of waste salt, such as nanofiltration, is high cost and hard to popularize. In this study, the photoelectrocatalytic treatment of waste salt obtained from the glyphosate industry and its utilization as a raw material for chlor-alkali electrolysis are proved to be feasible. The waste salt consists mainly of NaCl, with ~ 1.31 wt% of organic impurities. A TiO2 nanotube electrode was employed for the photoelectrocatalytic treatment of brine with NaCl concentration of 270 g L−1 prepared from waste salt. After preliminary treatment, the total organic carbon content (TOC) of the waste salt brine was reduced to 50 mg L−1, with a removal ratio of 85%. It is able to meet the standard of refined brine in the chlor-alkali industry (TOC < 20 mg L−1) with further treatment. A study on the photoelectrocatalytic mechanism reveals that the main oxidative species contributing to the degradation are holes (h+) and chlorine active substances other than Cl∙ under the condition of high Cl− concentration. The organic impurities in the waste salt are poisonous to both the electrode and membrane in the process of chlor-alkali electrolysis, leading to an increase in the voltage. With photoelectrocatalytic treatment, most of the organic impurities can be removed so that the waste salt can be utilized as a raw material for chlor-alkali electrolysis.
Graphic Abstract











Similar content being viewed by others
References
Liu D, Liu Q, Zhang Y (2021) Research progress on zero discharge and resource utilization of industrial high-salt wastewater. CLEAN-Soil Air Water 49:2000410. https://doi.org/10.1002/clen.202000410
Lefebvre O, Moletta R (2006) Treatment of organic pollution in industrial saline wastewater: a literature review. Water Res 40:3671–3682. https://doi.org/10.1016/j.watres.2006.08.027
Wright NC, Shah SR, Amrose SE, Amos G, Winter V (2018) A robust model of brackish water electrodialysis desalination with experimental comparison at different size scales. Desalination 443:27–43. https://doi.org/10.1016/j.desal.2018.04.018
Ali ES, Habry K, Askalany AA, Diab MR, Alsaman AS (2017) Weather effect on a solar powered hybrid adsorption desalination-cooling system: a case study of Egypt’s climate. Appl Therm Eng 124:663–672. https://doi.org/10.1016/j.applthermaleng.2017.06.048
Shestakov KV, Firpo R, Bittino A, Comite A (2017) Preliminary study of electrodialysis with model salt solutions and industrial wastewater. In: Mannina G (ed) Frontiers in wastewater treatment and modelling. Springer, Cham, pp 656–661. https://doi.org/10.1007/978-3-319-58421-8_103
Sana A, Florian G, Daniel W, Joachim W, Joachim K, Sven UG, Latifa B (2019) Application of direct contact membrane distillation for saline dairy effluent treatment: performance and fouling analysis. Environ Sci Pollut Res Int 26:18979–18992. https://doi.org/10.1007/s11356-018-2475-3
Kim KH, Ihm SK (2011) Heterogeneous catalytic wet air oxidation of refractory organic pollutants in industrial wastewaters: a review. J Hazard Mater 186:16–34. https://doi.org/10.1016/j.jhazmat.2010.11.011
Muthukumar M, Sargunamani D, Selvakumar N (2005) Statistical analysis of the effect of aromatic, azo and sulphonic acid groups on decolouration of acid dye effluents using advanced oxidation processes. Dyes Pigm 65:151–158. https://doi.org/10.1016/j.dyepig.2004.07.012
Ribeiro JR, Nunes ML (2021) Recent trends and developments in Fenton processes for industrial wastewater treatment – A critical review. Environ Res 197:110957. https://doi.org/10.1016/j.envres.2021.110957
Güven G, Perendeci NA, Tanyolaç A (2014) Reaction kinetics on treatment of food industry wastewaters by electrochemical oxidation. Desalination Water Treat 52:37–39. https://doi.org/10.1080/19443994.2013.827780
Mclntyre HM, Hart ML (2021) Photocatalytic porous silica-based granular media for organic pollutant degradation in industrial waste-streams. Catalysts 11:258. https://doi.org/10.3390/catal11020258
Li G, An T, Chen J, Sheng G, Fu J, Chen F, Zhang S, Zhao H (2006) Photoelectrocatalytic decontamination of oilfield produced wastewater containing refractory organic pollutants in the presence of high concentration of chloride ions. J Hazard Mater 138:392–400. https://doi.org/10.1016/j.jhazmat.2006.05.083
Ye S, Chen Y, Yao X, Zhang J (2020) Simultaneous removal of organic pollutants and heavy metals in wastewater by photoelectrocatalysis: a review. Chemosphere 273:128503. https://doi.org/10.1016/j.chemosphere.2020.128503
Ge M, Cao C, Huang J, Li S, Zhang S, Deng S, Li Q, Zhang K, Lai Y (2016) Synthesis, modification, and photo/photoelectrocatalytic degradation applications of TiO2 nanotube arrays: a review. Nanatechnol Reviews 5:1–62. https://doi.org/10.1515/ntrev-2015-0049
Meijide J, Lama G, Pazos M, Sanromán MA, Dunlop PSM (2022) Ultraviolet-based heterogeneous advanced oxidation processes as technologies to remove pharmaceuticals from wastewater: an overview. J Environ Chem Eng 10:107630. https://doi.org/10.1016/j.jece.2022.107630
Zhang X, Ai Z, Jia F, Zhang L, Fan X, Zou Z (2007) Selective synthesis and visible-light photocatalytic activities of BiVO4 with different crystalline phases. Mater Chem Phys 103:162–167. https://doi.org/10.1016/j.matchemphys.2007.02.008
Zhu L, Bing N, Wang L, Jin H, Liao G, Wang L (2012) Self-assembled 3D porous flowerlike α-Fe2O3 hierarchical nanostructures: synthesis, growth mechanism, and their application in photocatalysis. Dalton Trans 41:2959–2965. https://doi.org/10.1039/c2dt11822j
Sergi GS, Enric B (2017) Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J Photochem Photobiology C: Photochem Reviews 31:1–35. https://doi.org/10.1016/j.jphotochemrev.2017.01.005
Li D, Fu Y (2020) Industrial waste salt conversion device and method. CN111790731A
Roy P, Berger S, Schmuki P (2011) TiO2 nanotubes: synthesis and applications. Angew Chem Int Ed 50:2904–2939. https://doi.org/10.1002/anie.201001374
Kong X, Pu J, Zhou H, Liu S, Li B, Deng Q et al (2012) Glyphosate circulating production method. CN103012474A
Pelegrini R, Peralta-Zamora P, Andrade AR, Reyes J, Durán N (1999) Electrochemically assisted photocatalytic degradation of reactive dyes. Appl Catal B Environ 22:83–90. https://doi.org/10.1016/S0926-3373(99)00037-5
Li D, Tong H, Zhang L (2013) Preparation of TiO2/ITO film by liquid phase deposition and its photoelectrocatalytic activity for degradation of 4-aminoantipyrine. Trans Nonferrous Met Soc China 23:3306–3311. https://doi.org/10.1016/S1003-6326(13)62868-X
GBT 12686 – 2017, Glyphosate technical
Macak JM, Tsuchiya H, Ghicov A, Yasuda K, Hahn R, Bauer S, Schmuki P (2007) TiO2 nanotubes: self-organized electrochemical formation, properties and applications. Curr Opin Solid State Mater Sci 11:3–18. https://doi.org/10.1016/j.cossms.2007.08.004
Liao W, Zhang Y, Zhang M, Murugananthan M, Yoshihara S (2013) Photoelectrocatalytic degradation of microcystin-LR using Ag/AgCl/TiO2 nanotube arrays electrode under visible light irradiation. Chem Eng J 231:455–463. https://doi.org/10.1016/j.cej.2013.07.054
Ishibashi K, Fujishima A, Watanabe T, Hashimoto K (2000) Detection of active oxidative species in TiO2 photocatalysis using the fluorescence technique. Electrochem Commun 2:207–210. https://doi.org/10.1016/S1388-2481(00)00006-0
Li F, Peng X, Liu Y, Mei J, Sun L, Shen C, Ma C, Huang M, Wng Z, Sand W (2019) A chloride-radical-mediated electrochemical filtration system for rapid and effective transformation of ammonia to nitrogen. Chemosphere 229:383–391. https://doi.org/10.1016/j.chemosphere.2019.04.180
Qin W, Wang Y, Fang G, Wu T, Liu C, Zhou D (2016) Evidence for the generation of reactive oxygen species from hydroquinone and benzoquinone: roles in arsenite oxidation. Chemosphere 150:71–78. https://doi.org/10.1016/j.chemosphere.2016.01.119
Zhang F, Pi Y, Cui J, Yang Y, Zhang X, Guan N (2007) Unexpected selective photocatalytic reduction of Nitrite to Nitrogen on Silver-Doped Titanium Dioxide. J Phys Chem C 111:3756–3761. https://doi.org/10.1021/jp067807j
Shyam L, Thanapalan M (2014) The chlor-alkali process: work in Progress. Clean Technol Environ Policy 16:225–234. https://doi.org/10.1007/s10098-013-0630-6
Moussallem I, Jörissen J, Kunz U, Pinnow S, Turek T (2008) Chlor-alkali electrolysis with oxygen depolarized cathodes: history, present status and future prospects. J Appl Electrochem 38:1177–1194. https://doi.org/10.1007/s10800-008-9556-9
Zhou H, Zhang Y (2014) Enhanced electrochemical performance of manganese dioxide spheres deposited on a titanium dioxide nanotube arrays substrate. J Power Sources 272:866–879. https://doi.org/10.1016/j.jpowsour.2014.09.030
Zhou H, Zhang Y (2014) Electrochemically Self-Doped TiO2 nanotube arrays for supercapacitors. J Phys Chem C 118:5626–5636. https://doi.org/10.1021/jp4082883
QB/T 5270 – 2018. Salt for ion-exchange membrane caustic soda
Sun J, Guo Y, Wang Y, Cao D, Tian S, Xiao K, Mao R, Zhao X (2018) H2O2 assisted photoelectrocatalytic degradation of diclofenac sodium at g-C3N4/BiVO4 photoanode under visible light irradiation. Chem Eng J 332:312–320. https://doi.org/10.1016/j.cej.2017.09.041
Shukla R, Madras G (2014) Cyclic reaction network modeling for the kinetics of photoelectrocatalytic degradation. J Environ Chem Eng 2:780–787. https://doi.org/10.1016/j.jece.2014.01.024
Sökmen M, Özkan A (2002) Decolourising textile wastewater with modified titania: the effects of inorganic anions on the photocatalysis. J Photochem Photobiology A: Chem 147:77–81. https://doi.org/10.1016/S1010-6030(01)00627-X
Polcaro AM, Palmas S, Renoldi F, Mascia M (1999) On the performance of Ti/SnO2 and Ti/PbO2 anodesin electrochemical degradation of 2-chlorophenolfor wastewater treatment. J Appl Electrochem 29:147–151. https://doi.org/10.1023/A:1003411906212
Yuan R, Ramjaun SN, Wang Z, Liu J (2011) Effects of chloride ion on degradation of Acid Orange 7 by sulfate radical-based advanced oxidation process: implications for formation of chlorinated aromatic compounds. J Hazard Mater 196:173–179. https://doi.org/10.1016/j.jhazmat.2011.09.007
Kiwi J, Lopez A, Nadtochenko V (2000) Mechanism and kinetics of the OH-radical intervention during fenton oxidation in the presence of a significant amount of radical scavenger (Cl-). Environ Sci Technol 34:2162–2168. https://doi.org/10.1021/es991406i
Liu D, Ma C, Gu G, Wang C, Chen X (2017) Removal of isopropyl ethylthionocarbamate from aqueous solution by oxidation. Desalination Water Treat 72:228–234. https://doi.org/10.5004/dwt.2017.20324
Tang J, Li M, Li Z (2019) Oxidation mechanisms of all kinds of active substances by hybrid photoelectrocatalytic treatment. China Environ Sci 39:2048–2054. https://doi.org/10.19674/j.cnki.issn1000-6923.2019.0246
Zheng H, Gao J, Wang S, Li H (2013) Fundamental scientific aspects of lithium batteries (VI)—Ionic transport in solids. Energy Storage Science and Technology 2:620–635. https://doi.org/10.3969/j.cnki.issn.2095-4239.2013.06.010
Acknowledgements
The authors acknowledge financial support from Chibi Research Institute for High-Quality Development.
Author information
Authors and Affiliations
Contributions
XH conceived of and supervised the project; HZ and HZ designed the experiments, analyzed the data, and wrote the paper; HZ and LT carried out the experiments; all authors commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, H., Zhou, H., Tang, L. et al. Photoelectrocatalytic treatment and resource utilization of industrial waste salt for chlor-alkali electrolysis. J Appl Electrochem 53, 963–975 (2023). https://doi.org/10.1007/s10800-022-01821-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01821-8