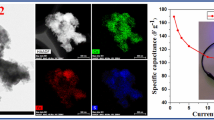
Abstract
Supercapattery is a hybrid renewable device that stores a significant amount of energy and delivers sufficient power together. In this paper, we used the hydrothermal technique for the synthesis of nickel-manganese sulfide (NiMnS) and carbon nanotube (CNT)-incorporated nickel-manganese sulfide (NiMnS/CNT). The surface and structural analyses were done using SEM and XRD. In a three-cell arrangement, NiMNS delivered the specific capacity of 685 Cg−1 at the current density of 1.9 Ag−1. The incorporation of CNT into NiMnS significantly improves the storage capacity. The NiMnS75/CNT25 composite delivered the specific capacity of 1188 Cg−1 at the current density of 2.8 Ag−1. The supercapattery device was designed using NiMnS75/CNT25 as the anode while activated carbon as the cathode. The supercapattery (NiMnS75/CNT25//AC) demonstrates an outstanding specific capacity of 105.9 Cg−1 at the current density of 0.6 Ag−1. The device (NiMnS75/CNT25//AC) provided a high power density of 1600.8 WKg−1 at an energy density of 9 WhKg−1. These results suggest NiMnS75/CNT25 as a more suitable electrode material for supercapattery applications.
Graphical Abstract









Similar content being viewed by others
References
Dubal DP et al (2018) Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem Soc Rev 47(6):2065–2129
Browne MP, Sofer Z, Pumera M (2019) Layered and two dimensional metal oxides for electrochemical energy conversion. Energy Environ Sci 12(1):41–58
Liu Z et al (2015) Flexible electronics based on inorganic nanowires. Chem Soc Rev 44(1):161–192
Niu Z et al (2013) Highly stretchable, integrated supercapacitors based on single-walled carbon nanotube films with continuous reticulate architecture. Adv Mater 25(7):1058–1064
Xu J et al (2013) Flexible asymmetric supercapacitors based upon Co9S8 nanorod//Co3O4@ RuO2 nanosheet arrays on carbon cloth. ACS Nano 7(6):5453–5462
Xie J et al (2013) Layer-by-layer β-Ni (OH) 2/graphene nanohybrids for ultraflexible all-solid-state thin-film supercapacitors with high electrochemical performance. Nano Energy 2(1):65–74
Tang Y et al (2016) Honeycomb-like mesoporous cobalt nickel phosphate nanospheres as novel materials for high performance supercapacitor. Electrochim Acta 190:118–125
Teng Z et al (2017) Bandgap engineering of ultrathin graphene-like carbon nitride nanosheets with controllable oxygenous functionalization. Carbon 113:63–75
Zhao Q et al (2017) Facile synthesis of Mn 3 [Co (CN) 6] 2·nH2O nanocrystals for high-performance electrochemical energy storage devices. Inorg Chem Front 4(3):442–449
Surendran S et al (2018) Carbon-enriched cobalt phosphide with assorted nanostructure as a multifunctional electrode for energy conversion and storage devices. ChemistrySelect 3(43):12303–12313
Pervez SA et al (2015) Anodic WO3 mesosponge@ carbon: a novel binder-less electrode for advanced energy storage devices. ACS Appl Mater Interfaces 7(14):7635–7643
Bruce PG, Freunberger SA, Hardwick LJ, Tarascon J-M (2011) Nat Mater 11:172
Pervez SA et al (2019) Fabrication of a dendrite-free all solid-state li metal battery via polymer composite/garnet/polymer composite layered electrolyte. Adv Mater Interfaces 6(11):1900186
Zheng JP (2009) High energy density electrochemical capacitors without consumption of electrolyte. J Electrochem Soc 156(7):A500
Tan K et al (2012) Stability and hydrolyzation of metal organic frameworks with paddle-wheel SBUs upon hydration. Chem Mater 24(16):3153–3167
Shimizu W et al (2013) Development of a 4.2 V aqueous hybrid electrochemical capacitor based on MnO2 positive and protected Li negative electrodes. J Power Sour 241:572–577
Iqbal MZ et al (2020) Co-MOF/polyaniline-based electrode material for high performance supercapattery devices. Electrochim Acta 346:136039
Kim H et al (2020) Fabrication of an ingenious metallic asymmetric supercapacitor by the integration of anodic iron oxide and cathodic nickel phosphide. Appl Surf Sci 511:145424
Chen J et al (2018) Integrated paper electrodes derived from cotton stalks for high-performance flexible supercapacitors. Nano Energy 53:337–344
Balamurugan J et al (2017) Hierarchical design of Cu 1–x Ni x S nanosheets for high-performance asymmetric solid-state supercapacitors. J Mater Chem A 5(37):19760–19772
Priyadharsini N et al (2018) Effect of chelating agent on the sol–gel thermolysis synthesis of LiNiPO4 and its electrochemical properties for hybrid capacitors. J Phys Chem Solids 119:183–192
Lin L-Y et al (2019) Synthesizing Ni-based ternary metal compounds for battery-supercapacitor hybrid devices with and without using nickel precursors. Mater Sci Semicond Process 98:81–89
Sahoo S et al (2018) Incorporation of carbon quantum dots for improvement of supercapacitor performance of nickel sulfide. ACS Omega 3(12):17936–17946
Tang Y, Chen T, Yu S (2015) Morphology controlled synthesis of monodispersed manganese sulfide nanocrystals and their primary application in supercapacitors with high performances. Chem Commun 51(43):9018–9021
Lee H-W et al (2014) Manganese hexacyanomanganate open framework as a high-capacity positive electrode material for sodium-ion batteries. Nat Commun 5(1):1–6
Sakib MN et al (2021) A review of recent advances in manganese-based supercapacitors. J Energy Storage 44:103322
Cao J et al (2021) Synthesis of mesoporous nickel-cobalt-manganese sulfides as electroactive materials for hybrid supercapacitors. Chem Eng J 405:126928
Sim Y et al (2022) Fluorine-doped graphene oxide prepared by direct plasma treatment for supercapacitor application. Chem Eng J 428:132086
Xia C et al (2022) A sulfur self-doped multifunctional biochar catalyst for overall water splitting and a supercapacitor from Camellia japonica flowers. Carbon Energy 4:491–505
Hu L et al (2009) Highly conductive paper for energy-storage devices. Proc Natl Acad Sci USA 106(51):21490–21494
Chen W et al (2011) High-performance nanostructured supercapacitors on a sponge. Nano Lett 11(12):5165–5172
Peng L et al (2015) Nickel sulfide nanoparticles synthesized by microwave-assisted method as promising supercapacitor electrodes: an experimental and computational study. Electrochim Acta 182:361–367
Faisal MM et al (2022) Redox-active anomalous electrochemical performance of mesoporous nickel manganese sulfide nanomaterial as an anode material for supercapattery devices. Ceram Int 48(19):28565–28577
Vijayakumar S, Nagamuthu S, Muralidharan G (2013) Supercapacitor studies on NiO nanoflakes synthesized through a microwave route. ACS Appl Mater Interfaces 5(6):2188–2196
Omar FS et al (2017) Binary composite of polyaniline/copper cobaltite for high performance asymmetric supercapacitor application. Electrochim Acta 227:41–48
Iqbal MZ et al (2019) Ultrasonication-assisted synthesis of novel strontium based mixed phase structures for supercapattery devices. Ultrason Sonochem 59:104736
Onoda H, Sakumura T (2011) Synthesis and pigmental properties of nickel phosphates by the substitution with tetravalent cerium cation. Mater Sci Appl 2(11):1578–1583
Wang Y et al (2009) Sn@ CNT and Sn@ C@ CNT nanostructures for superior reversible lithium ion storage. Chem Mater 21(14):3210–3215
Frackowiak E et al (2000) Supercapacitor electrodes from multiwalled carbon nanotubes. Appl Phys Lett 77(15):2421–2423
Macdonald JR, Barsoukov E (2018) Impedance spectroscopy: theory, experiment, and applications. John Wiley & Sons, New York
Niu C et al (1997) High power electrochemical capacitors based on carbon nanotube electrodes. Appl Phys Lett 70(11):1480–1482
Duraisamy N et al (2015) Investigation on structural and electrochemical properties of binder free nanostructured nickel oxide thin film. Mater Lett 161:694–697
Zhao G et al (2020) High-performance and flexible all-solid-state hybrid supercapacitor constructed by NiCoP/CNT and N-doped carbon coated CNT nanoarrays. J Colloid Interface Sci 572:151–159
Jain R et al (2020) MnO2@ Polyaniline-CNT-boron-doped graphene as a freestanding binder-free electrode material for supercapacitor. J Mater Sci: Mater Electron 31(11):8385–8393
Wang J et al (2020) Enhancing the electrical conductivity of PP/CNT nanocomposites through crystal-induced volume exclusion effect with a slow cooling rate. Compos B Eng 183:107663
Fujigaya T, Nakashima N (2015) Non-covalent polymer wrapping of carbon nanotubes and the role of wrapped polymers as functional dispersants. Sci Technol Adv Mater 16(2):024802
Gu J et al (2020) MOF-derived Ni-doped CoP@ C grown on CNTs for high-performance supercapacitors. Chem Eng J 385:123454
Hu Z et al (2015) High specific capacitance of polyaniline/mesoporous manganese dioxide composite using KI-H2SO4 electrolyte. Polymers 7(10):1939–1953
Iqbal MF et al (2017) High specific capacitance and energy density of synthesized graphene oxide based hierarchical Al2S3 nanorambutan for supercapacitor applications. Electrochim Acta 246:1097–1103
Jia H et al (2019) Designing oxygen bonding between reduced graphene oxide and multishelled Mn3 O4 hollow spheres for enhanced performance of supercapacitors. J Mater Chem A 7(12):6686–6694
Farbod F et al (2019) Synthesis of a porous interconnected nitrogen-doped graphene aerogel matrix incorporated with ytterbium oxide nanoparticles and its application in superior symmetric supercapacitors. Electrochim Acta 306:480–488
Akinwolemiwa B, Peng C, Chen GZ (2015) Redox electrolytes in supercapacitors. J Electrochem Soc 162(5):A5054
Ghadimi LS et al (2018) Novel nanocomposite of MnFe2O4 and nitrogen-doped carbon from polyaniline carbonization as electrode material for symmetric ultra-stable supercapacitor. Electrochim Acta 282:116–127
Halper, M. and J. Ellenbogen, Supercapacitors: A Brief Overview The MITRE Corporation, 2006, McLean Virginia
Iqbal MZ et al (2020) Capacitive and diffusion-controlled mechanism of strontium oxide based symmetric and asymmetric devices. J Energy Storage 27:101056
Chen K, Xue D (2017) Colloidal paradigm in supercapattery electrode systems. Nanotechnology 29(2):024003
Zhang Y et al (2019) Cobalt-nickel silicate hydroxide on amorphous carbon derived from bamboo leaves for hybrid supercapacitors. Chem Eng J 375:121938
Manikandan R et al (2019) Self-coupled nickel sulfide@ nickel vanadium sulfide nanostructure as a novel high capacity electrode material for supercapattery. Appl Surf Sci 497:143778
Shahabuddin S et al (2019) Polyaniline-SrTiO3 nanocube based binary nanocomposite as highly stable electrode material for high performance supercapaterry. Ceram Int 45(9):11428–11437
Babu IM, William JJ, Muralidharan G (2019) Hierarchical β-Co (OH) 2/CoO nanosheets: an additive-free synthesis approach for supercapattery applications. Ionics 25(5):2437–2444
Numan A et al (2020) Facile sonochemical synthesis of 2D porous Co3O4 nanoflake for supercapattery. J Alloy Compd 819:153019
Lee CC et al (2017) An enhanced performance of hybrid supercapacitor based on polyaniline-manganese phosphate binary composite. J Solid State Electrochem 21(11):3205–3213
Ma L et al (2019) A 3D self-supported coralline-like CuCo2S4@ NiCo2S4 core–shell nanostructure composite for high-performance solid-state asymmetrical supercapacitors. Nanotechnology 30(25):255603
Iqbal J et al (2020) Ternary nanocomposite of cobalt oxide nanograins and silver nanoparticles grown on reduced graphene oxide conducting platform for high-performance supercapattery electrode material. J Alloy Compd 821:153452
Acknowledgements
The Higher Education Commission (HEC) of Pakistan financed this research under the National Research Program for Universities (NRPU), project number HEC/R&D/NRPU/2017/7876. The authors would like to thank Riphah International University for sponsoring this research under the project number Riphah-ORIC-21-22/FEAS-04.
Author information
Authors and Affiliations
Contributions
MW, MHK, and AMA worked on experiment, data collection, analysis, and interpretation of the results. MWI, MHK, and AMA performed the calculation and wrote the manuscript. MWI, MHK, AMA, HH, HAA, and SA helped with the calculation process and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no conflict of interest.
Ethical approval
The submitted work should be original and should not have been published elsewhere in any form or language.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iqbal, M.W., Khan, M.H., Afzal, A.M. et al. Incorporation of carbon nanotubes in sulfide-based binary composite to enhance the storage performance of supercapattery devices. J Appl Electrochem 53, 949–962 (2023). https://doi.org/10.1007/s10800-022-01820-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01820-9