Abstract
The strategy of obtaining value-added products from electrochemical reduction of CO2 is promising and advantageous over other conventional methods. The present work uses an electrodeposition method to prepare electro-decorated Sn on Cu foam. The physicochemical and morphology analyses were studied using XRD, XPS, and FESEM. The electrocatalytic reduction of CO2 of the prepared electrocatalyst was successfully demonstrated in 0.5-M KHCO3 electrolyte in two different cell designs—a conventional H-type cell and an improved cell design by the two parallel-connected electrodes. The formate product formed during CO2 reduction was identified using NMR, and the faradaic efficiency at different potentials was calculated. The prepared electro-decorated Sn@Cu foam electrode displayed exceptional stability under CO2 reduction conditions over a period of 20-h duration in a conventional method. We extended our work for continuous CO2 electroreduction with parallel-connected electrodes to obtain formate at ambient temperature and pressure. The parallel mode was adopted to maximize the active sites so as to achieve maximum product concentration. The formate concentration of 6.12 g L−1 was achieved with electro-decorated Sn@Cu foam electrode on continuous CO2 reduction in a batch mode setup. The proposed approach of producing formate via batch mode CO2 reactor at a larger scale would open vistas for the industrialization (scale-up) of the process.
Graphical abstract
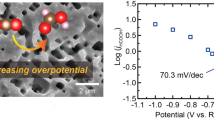
The present work is focused on the preparation of electro-decorated Sn@Cu foam using electrodeposition methods. Electroreduction of CO2 was carried out using the tin on Cu electrocatalyst. We observed the selective formation of formate. With the improved cell design, we can achieve the formate concentration of 6.12 g L−1 on 20 h of continuous CO2 electrolysis.
















Similar content being viewed by others
References
Rogelj J, den Elzen M, Hohne N, Fransen T, Fekete H, Winkler H, Chaeffer RS, Ha F, Riahi K, Meinshausen M (2016) Paris agreement climate proposals need a boost to keep warming well below 2 degrees C. Nature 534:631–639
Seoane B, Coronas J, Gascon I, Benavides ME, Karvan O, Caro J, Kapteijn F, Gascon J (2015) Metal-organic framework based mixed matrix membranes: a solution for highly efficient CO2 capture. Chem Soc Rev 44:2421–2454
Sanz-Perez ES, Murdock CR, Didas SA, Jones CW (2016) Direct capture of CO2 from ambient air. Chem Rev 116:11840–11876
Gao W, Zhou T, Gao Y, Louis B, O’Hare D, Wang Q (2017) Molten salts-modified MgO-based adsorbents for intermediate-temperature CO2 capture: a review. J Energy Chem 26:830–838
Alcalde J, Flude S, Wilkinson M, Johnson G, Edlmann K, Bond CE, Scott V, Gilfillan SMV, Ogaya X, Haszeldine RS (2018) Estimating geological CO2 storage security to deliver on climate mitigation. Nat Commun 9:13
Aminu MD, Nabavi SA, Rochelle CA, Manovic V (2017) A review of developments in carbon dioxide storage. Appl Energy 208:1389–1419
Li Q, Fu J, Zhu W, Chen Z, Shen B, Wu L, Xi Z, Wang T, Lu G, Zhu JJ, Sun S (2017) Tuning Sn-catalysis for electrochemical reduction of CO2 to CO via the core/shell Cu/SnO2 structure. J Am Chem Soc 139:4290–4293
Zhang L, Zhao ZJ, Wang T, Gong JL (2018) Nano-designed semiconductors for electro- and photoelectro-catalytic conversion of carbon dioxide. Chem Soc Rev 47:5423–5443
Moller T, Ju W, Bagger A, Wang X, Luo F, Ngo Thanh T, Varela AS, Rossmeisl J, Strasser P (2019) Efficient CO2 to CO electrolysis on solid Ni–N–C catalysts at industrial current densities. Energy Environ Sci 12:640–647
Zheng X, Ji Y, Tang J, Wang J, Liu B, Steinrück HG, Lim K, Li Y, Toney MF, Chan K, Cui Y (2019) Theory-guided Sn/Cu alloying for efficient CO2 electroreduction at low overpotentials. Nat Catal 2:55–61
Dong ZD, Long LJ, Zhang QS (2016) Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Adv Mater 28:3423–3452
Qiao J, Liu Y, Hong F, Zhang J (2014) A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem Soc Rev 43:631–675
Wu J, Risalvato FG, Ke FS, Pellechia PJ, Zhou XD (2012) Electrochemical reduction of carbon dioxide I. Effects of the electrolyte on the selectivity and activity with Sn electrode. J Electrochem Soc 159:F353–F359
Gu J, Heroguel F, Luterbacher J, Hu X (2018) Densely packed, ultra small SnO nanoparticles for enhanced activity and selectivity in electrochemical CO2 reduction. Angew Chem Int Ed 57:2943–2947
Yu J, Liu H, Song S, Wang Y, Tsiakaras P (2017) Electrochemical reduction of carbon dioxide at nanostructured SnO2/carbon aerogels: the effect of tin oxide content on the catalytic activity and formate selectivity. Appl Catal A 545:159–166
Tan D, Lee W, Kim YE, Ko YN, Youn MH, Jeon YE, Hong J, Jeong SK, Park KT (2020) SnO2/ZnO composite hollow nanofiber electrocatalyst for efficient CO2 reduction to formate. ACS Sustain Chem Eng 8:10639–10645
Lai Q, Yuan W, Huang W, Yuan G (2020) Sn/SnOx electrode catalyst with mesoporous structure for efficient electroreduction of CO2 to formate. Appl Surf Sci 508:145221
Li J, Jiao J, Zhang H, Zhu P, Ma H, Xiao CCH, Lu Q (2020) Two-dimensional SnO2 nanosheets for efficient carbon dioxide electroreduction to formate. ACS Sustain Chem Eng 8:4975–4982
Pavithra K, Senthil Kumar SM (2020) Embedding oxygen vacancies at SnO2–CNT surfaces via a microwave polyol strategy towards effective electrocatalytic reduction of carbon-dioxide to formate. Catal Sci Technol 10:1311–1322
Rasul S, Pugnant A, Xiang H, Fontmorin JM, Yu EH (2019) Low cost and efficient alloy electrocatalysts for CO2 reduction to formate. J CO2 Util. https://doi.org/10.1016/j.jcou.2019.03.016
Chen C, Pang Y, Zhang F, Zhong J, Zhang B, Cheng Z (2018) Sharp Cu@Sn nanocones on Cu foam for highly Selective and efficient electrochemical reduction of CO2 to formate. J Mater Chem A 6:19621–19630
Zhang M, Zhang Z, Zhao Z, Huang H, Anjum DH, Wang D, He J, Huang K (2021) tunable selectivity for electrochemical CO2 reduction by bimetallic Cu−Sn catalysts: elucidating the roles of Cu and Sn. ACS Catal 11:11103–11108
Lim J, Garcia-Esparza AT, Lee JW, Kang G, Shin SS, Jeona S, Lee H (2022) Electrodeposited Sn–Cu@Sn dendrites for selective electrochemical CO2 reduction to formic acid. Nanoscale 14:9297
Rabiee H, Zhang X, Ge L, Hu S, Li M, Smart S, Zhu Z, Yuan Z (2020) Tuning the Product selectivity of the Cu hollow fiber gas diffusion electrode for efficient CO2 reduction to formate by controlled surface Sn electrodeposition. ACS Appl Mater Interfaces 12:21670–21681
Lim J, Kang PW, Jeon SS, Lee H (2020) Electrochemically deposited Sn catalysts with dense tips on a gas diffusion electrode for electrochemical CO2 reduction. J Mater Chem A 8:9032–9038
Zhao C, Wang J (2016) Electrochemical reduction of CO2 to formate in aqueous solution using electro-deposited Sn catalysts. Chem Eng J 293:161–170
Liang S, Altaf N, Huang L, Gao Y, Wang Q (2020) Electrolytic cell design for electrochemical CO2 reduction. J CO2 Util. https://doi.org/10.1016/j.jcou.2019.09.007
Choi SY, Jeong SK, Kim HJ, Baek IH, Park KT (2016) Electrochemical reduction of carbon dioxide to formate on tin–lead alloys. ACS Sustain Chem Eng 4:1311–1318
Sen S, Brown M, Leonard M, Brushett FR (2019) Electroreduction of carbon dioxide to formate at high current densities using tin and tin oxide gas diffusion electrodes. J Appl Electrochem 49:917–928
Baruch MF, Pander JE, White JL, Bocarsly AB (2015) Mechanistic Insights into the reduction of CO2 on tin electrodes using in situ ATR-IR spectroscopy. ACS Catal 5:3148–3156
Bienen F, Kopljar D, Geiger S, Wagner N, Friedrich KA (2020) Investigation of CO2 electrolysis on tin foil by electrochemical impedance spectroscopy. ACS Sustain Chem Eng 8:5192–5199
Li F, Chen L, Knowles GP, MacFarlane DR, Zhang J (2017) Hierarchical mesoporous SnO2 nanosheets on carbon cloth: a robust and flexible electrocatalyst for CO2 reduction with high efficiency and selectivity. Angew Chem Int Ed 56:505–509
Daiyan R, Lu X, Saputera WH, Ng YH, Amal R (2018) Highly selective reduction of CO2 to formate at low overpotentials achieved by a mesoporous tin oxide electrocatalyst. ACS Sustain Chem Eng 6:1670–1679
Acknowledgements
The authors acknowledge CSIR, New Delhi, India, for funding through Catalysis for Sustainable Development (HCP0009). We also recognize the Central Instrumental Facility, CSIR-CECRI, for their support. CSIR-CECRI manuscript reference number: CECRI/PESVC/Pubs/2022-025.
Author information
Authors and Affiliations
Contributions
SR conceived the idea and planned the research work; RS made electrocatalytic material; SMS and MA and KP performed the initial analysis of catalyst; PA characterized and analyzed and performed the electrochemical experiments; MG assisted the continuous cell operation; PA and SR wrote the manuscript. KP and SMS reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
This work is dedicated to Manickam Anbu Kulandainathan (1964–2021).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandiarajan, A., Sekar, R., Pavithra, K. et al. Highly selective electrochemical CO2 reduction to formate using Sn@Cu electrocatalyst. J Appl Electrochem 53, 1033–1042 (2023). https://doi.org/10.1007/s10800-022-01815-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01815-6