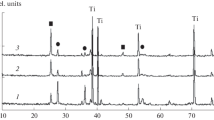
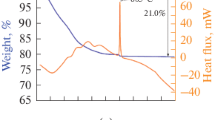
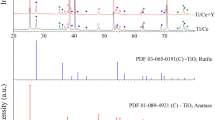
Abstract
Ti/TiO2-Sb-SbOx multifunctional sensors were obtained by plasma electrolytic oxidation in the anodic–cathodic mode. The resulting films contain TiO2 and up to 5 at.% Sb in the form of Sb0 and Sb6O13. Electrode functions E vs pH are linear in the range of pH 2 ÷ 10 with slopes of 68 ± 6 and 48 ± 2 mV·pH−1 for sensors formed at iC/iA = 1 and 1.2, respectively. The Ti/TiO2-Sb-SbOx sensor obtained at iC/iA = 1.2, despite its low response, shows results similar to the glass electrode in acid–base titration and can be used to accurately determine the alkalinity of natural and technogenic waters. The possibility of application of such sensors in potentiometric acid–base, redox, complexometric, and precipitation chemical reactions was revealed. The multifunctionality of the Ti/TiO2-Sb-SbOx sensor makes it possible to analyze aqueous solutions of complex composition using the same electrode.
Graphical abstract











Similar content being viewed by others
References
Roberts EJ, Fenwick F (1928) The antimony-antimony trioxide electrode and its use as a measure of acidity. J Am Chem Soc 50:2125–2147. https://doi.org/10.1021/ja01395a010
Stock JT, Purdy WC, Garcia LM (1958) The antimony-antimony oxide electrode. Chem Rev 58:611–626. https://doi.org/10.1021/cr50022a001
Głab S, Hulanicki A, Edwall G, Ingman F (1989) Metal-metal oxide and metal oxide electrodes as pH Sensors. Crit Rev Anal Chem 21:29–47. https://doi.org/10.1080/10408348908048815
Edwall G (1978) Improved antimony-antimony (III) oxide pH electrodes. Med Biol Eng Comput 16:661–669. https://doi.org/10.1007/BF02442445
Fog A, Buck RP (1984) Electronic semiconducting oxides as pH sensors. Sens Actuators 5:137–146. https://doi.org/10.1016/0250-6874(84)80004-9
Kurzweil P (2009) Metal oxides and ion-exchanging surfaces as pH sensors in liquids: state-of-the-art and outlook. Sensors 9:4955–4985. https://doi.org/10.3390/s90604955
Horton BE, Schweitzer S, DeRouin AJ, Ong KG (2011) A varactor-based, inductively coupled wireless pH sensor. IEEE Sens J 11:1061–1066. https://doi.org/10.1109/JSEN.2010.2062503
Manjakkal L, Szwagierczak D, Dahiya R (2020) Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog Mater Sci 109:100635. https://doi.org/10.1016/j.pmatsci.2019.100635
Neupane S, Mishra D, Nakarmi KB, Gupta DK, Yadav RJ, Yadav AP (2021) Preparation of antimony oxide in different media and its effect on the pH measurement. Anal Bioanal Electrochem 13:127–138
Ghalwa NA, Hamada M, Abu-Shawish HM, Swareh AA, Askalany MA, Siam T (2012) Using of Ti/Co3O4/PbO2/(SnO2+Sb2O3) modified electrode as indicator electrode in potentiometric and conductometric titration in aqueous solution. J Electroanal Chem 664:7–13. https://doi.org/10.1016/j.jelechem.2011.10.005
Hamada M (2014) Development of Ti/PbO2/Sb2O3 electrode as indicator electrode for pH measurements and conductometric titrations in aqueous solutions. Int J Pharm Bio Sci 5:50–65
Vasilyeva MS, Rudnev VS, Zabudskaya NE, Ustinov AYu, Zasukhina LA, Marinina GI (2020) Preparation and study of Ti/TiO2, SbOx pH electrodes. J Anal Chem 75:246–253. https://doi.org/10.1134/S1061934820020173
Serrano NM, Díaz-Cruz J, Ariño C, Esteban M (2016) Antimony-based electrodes for analytical determinations. Trends Anal Chem 77:203–213. https://doi.org/10.1016/j.trac.2016.01.011
Lakhdar MH, Smida YB, Amlouk M (2016) Synthesis, optical characterization and DFT calculations of electronic structure of Sb2O3 films obtained by thermal oxidation of Sb2S3. J Alloys Compd 681:197–204. https://doi.org/10.1016/j.jallcom.2016.04.026
Tan Y, Chen L, Chen H, Hou Q, Chen X (2018) Synthesis of a symmetric bundle-shaped Sb2O3 and its application for anode materials in lithium ion batteries. Mater Lett 212:103–106. https://doi.org/10.1016/j.matlet.2017.10.080
Han Q, Sheng Y, Han Z, Li X, Zhang W, Li Y, Zhang X (2020) Metallic Sb nanoparticles embedded into a yolk–shell Sb2O3@TiO2 composite as anode materials for lithium ion batteries. New J Chem 44:13430. https://doi.org/10.1039/c9nj05947d
Bryngelsson H, Eskhult J, Nyholm L, Herranen M, Alm O, Edström K (2007) Electrodeposited Sb and Sb/Sb2O3 nanoparticle coatings as anode materials for Li-ion batteries. Chem Mater 19:1170–1180. https://doi.org/10.1021/cm0624769
Bryngelsson H, Eskhult J, Edström K, Nyholm L (2007) Electrodeposition and electrochemical characterization of thick and thin coatings of Sb and Sb/Sb2O3 particles for Li ion battery anodes. Electrochim Acta 53:1062–1073. https://doi.org/10.1016/j.electacta.2007.02.009
Schutz RW, Thomas DE (2005) Corrosion of titanium and titanium alloys. In: Cramer SD and Covino, Jr. BS (eds) Corrosion: Materials. Vol 13B, ASM Handbook, ASM International, pp. 252–299
Chou JC, Liu CH, Chen CC (2011) Electrochromic property of sol-gel derived TiO2 thin film for pH sensor. IFMBE Proc 35:69–72. https://doi.org/10.1007/978-3-642-21729-6_21
Shin PK (2003) The pH-sensing and light-induced drift properties of titanium dioxide thin films deposited by MOCVD. Appl Surf Sci 214:214–221. https://doi.org/10.1016/S0169-4332(03)00340-4
Zhao R, Xu M, Wang J, Chen G (2010) A pH sensor based on the TiO2 nanotube array modified Ti electrode. Electrochim Acta 55:5647–5651. https://doi.org/10.1016/j.electacta.2010.04.102
Simić M, Manjakkal L, Zaraska K, Stojanović GM, Dahiya R (2016) TiO2 based thick film pH sensor. IEEE Sens J 17:248–255. https://doi.org/10.1109/JSEN.2016.2628765
Marinina GI, Reznik MF, Tyrin VI, Gordienko PS (1996) Electroanalytical properties of some oxide film electrodes. J Analyt Chem 51:896–899
Marinina GI, Vasilyeva MS, Lapina AS, Ustinov AYu, Rudnev VS (2013) Electroanalytical properties of metal–oxide electrodes formed by plasma electrolytic oxidation. J Electroan Chem 689:262–268. https://doi.org/10.1016/j.jelechem.2012.10.032
Vasilyeva MS, Rudnev VS, Arefieva OD, Lapina AS, Plyusnina VI, Marinina GI (2016) Ti/TiO2 indicator electrodes formed by plasma electrolytic oxidation for potentiometric analysis. Int J Environ Anal Chem 96:1128–1144. https://doi.org/10.1080/03067319.2016.1243241
Vasilyeva MS, Lukiyanchuk IV, Ustinov AY, Shchitovskaya EV, Marinina GI (2020) Anodic-cathodic formation of pH-sensitive TiO2-MoOx films on titanium. J Electroanal Chem 873:114388. https://doi.org/10.1016/j.jelechem.2020.114388
Kaseem M, Fatimah S, Nashrah N, Ko YG (2021) Recent progress in surface modification of metals coated by plasma electrolytic oxidation: principle, structure, and performance. Prog Mater Sci 117:100735. https://doi.org/10.1016/j.pmatsci.2020.100735
Aliofkhazraei M, Macdonald DD, Matykina E, Parfenov EV, Egorkin VS, Curran JA, Troughton SC, Sinebryukhov SL, Gnedenkov SV, Lampke T, Simchen F, Nabavi HF (2021) Review of plasma electrolytic oxidation of titanium substrates: Mechanism, properties, applications and limitations. Appl Surf Sci Adv 51:100121. https://doi.org/10.1016/j.apsadv.2021.100121
Grigoriev SN, Kondratsky I, Krit BL, Ludin VB, Medvetskova VM, Morozova NV, Suminov IV, Apelfeld AV, Wu RZ (2022) Protective and thermophysical characteristics of plasma electrolytic coatings on the ultralight magnesium alloy. J Eng Mater Technol Trans ASME 144:021006. https://doi.org/10.1115/1.4052718
Abbasi S, Mahboob A, Bakhtiari Zamani H, Bilesan MR, Repo E, Hakimi A (2022) The tribological behavior of nanocrystalline TiO2 coating produced by plasma electrolytic oxidation. J Nanomater. https://doi.org/10.1155/2022/5675038
Molaei M, Fattah-Alhosseini A, Nouri M, Mahmoodi P, Navard SH, Nourian A (2022) Enhancing cytocompatibility, antibacterial activity and corrosion resistance of PEO coatings on titanium using incorporated ZrO2 nanoparticles. Surf Interfaces 30:101967. https://doi.org/10.1016/j.surfin.2022.101967
Kostelac L, Pezzato L, Settimi AG, Franceschi M, Gennari C, Brunelli K, Rampazzo C, Dabalà M (2022) Investigation of hydroxyapatite (HAP) containing coating on grade 2 titanium alloy prepared by plasma electrolytic oxidation (PEO) at low voltage. Surf Interfaces 30:101888. https://doi.org/10.1016/j.surfin.2022.101888
Dong S, Wang J, Tang X, Li J, Zhang X, Liu B (2021) Low-temperature and stable CO oxidation of in-situ grown monolithic Mn3O4/TiO2 catalysts. J Alloys Compd 855:157444. https://doi.org/10.1016/j.jallcom.2020.157444
Stojadinović S, Radić N, Tadić N, Vasilić R, Tsanev A (2022) TiO2/Bi2O3 coatings formed by plasma electrolytic oxidation of titanium for photocatalytic applications. J Mater Sci: Mater Electron 33:4467–4481. https://doi.org/10.1007/s10854-021-07637-0
Mamaev AI, Dolgova YN, Yeltsov AA, Plekhanov GV, Ryabikov AE, Baranova TA, Mamaeva VA (2020) Heterogeneous metal oxide coatings with magnetoactive nickel, cobalt, and iron phases formed by the method of pulsed microplasma oxidation for radiation absorption in the middle and near-IR regions. Russ Phys J 63:1265–1276. https://doi.org/10.1007/s11182-020-02149-6
Xin SG, Song LX, Zhao RG, Hu XF (2006) Influence of cathodic current on composition, structure and properties of Al2O3 coatings on aluminum alloy prepared by micro-arc oxidation process. Thin Solid Films 515:326–332. https://doi.org/10.1016/j.tsf.2005.12.087
Sah SP, Tsuji E, Aoki Y, Habazaki H (2012) Cathodic pulse breakdown of anodic films on aluminium in alkaline silicate electrolyte - understanding the role of cathodic half-cycle in AC plasma electrolytic oxidation. Corrosion Sci 55:90–96. https://doi.org/10.1016/j.corsci.2011.10.007
Rogov AB, Huang Y, Shore D, Matthews A, Yerokhin A (2021) Toward rational design of ceramic coatings generated on valve metals by plasma electrolytic oxidation: The role of cathodic polarization. Ceram Int 47:34137–34158. https://doi.org/10.1016/j.ceramint.2021.08.324
Gebarowski W, Pietrzyk S (2013) Influence of the cathodic pulse on the formation and morphology of oxide coatings on aluminium, produced by plasma electrolytic oxidation. Arch Metal Mater 58:241–245. https://doi.org/10.2478/v10172-012-0180-7
Wojtas R (1975) Complexes of antimony(III). Part I. Polarographic studies on complex formation in systems: Sb(ClO4)3–L–H2O (L= CHO2-, C2H3O2-, C3H5O2-, C3H2O42-, C4H4O42-, C5H6O42-, C4H4O52-, and C4H4O62-. Rocz Chem 49:1231–1237. https://doi.org/10.1002/CHIN.197548366
Rudnev VS (2007) Growth of anodic oxide layers under electric discharge conditions. Protect Met 43:275–280. https://doi.org/10.1134/S0033173207030125
Luo Q, Cai QZ, He J, Li XW, Chen XD, Pan ZH, Li YJ (2014) A novel way to prepare visible-light-responsive WO3/TiO2 composite film with high porosity. Int J Appl Ceram Technol 11:254–262. https://doi.org/10.1111/ijac.12062
Rudnev VS, Adigamova MV, Lukiyanchuk IV, Ustinov AYu, Tkachenko IA, Kharitonskii PV, Frolov AM, Morozova VP (2012) The effect of the conditions of formation on ferromagnetic properties of iron-containing oxide coatings on titanium. Prot Met Phys Chem Surf 48:543–552. https://doi.org/10.1134/S2070205112050097
Voigt K, Heubner C, Liebmann T, Matthey B, Weiser M, Schneider M, Michaelis A (2020) Electrodeposition of versatile nanostructured Sb/Sb2O3 microcomposites: a parameter study Adv. Mater Interfaces. https://doi.org/10.1002/admi.202000004
Vovna VI, Gnedenkov SV, Gordienko PS, Kuznetsov MV, Sinebryukhov SL, Cherednichenko AI, Khrisanfova OA (1998) Surface layers produced on titanium by the microarc oxidation: An X-ray diffractometry study. Russ J Electrochem 34:1090–1093
Rudnev VS, Vaganov-Vil’kins AA, Ustinov AY, Nedozorov PM (2011) Carbon in oxide layers formed under electric discharge conditions. Prot Met Phys Chem 47:330–338. https://doi.org/10.1134/S2070205111030130
Gnedenkov SV, Sinebryukhov SL, Zavidnaya AG, Egorkin VS (2014) Composite hydroxyapatite–PTFE coatings on Mg–Mn–Ce alloy for resorbable implant applications via a plasma electrolytic oxidation based route. J Taiwan Inst Chem Eng 45:3104–3109. https://doi.org/10.1016/j.jtice.2014.03.022
Sanjinés R, Tang H, Berger H, Gozzo F, Lévy MG (1994) Electronic structure of anatase TiO2 oxide. J Appl Phys 75:2945. https://doi.org/10.1063/1.356190
Delobel R, Baussart H, Leroy JM, Grimblot J, Gengembre L (1983) X-ray photoelectron spectroscopy study of uranium and antimony mixed metal-oxide catalysts. J Chem Soc Faraday Trans 79:879–891. https://doi.org/10.1039/F19837900879
Wang M, Ha Y (2007) An electrochemical approach to monitor pH change in agar media during plant tissue culture. Biosens Bioelectron 22:2718–2723. https://doi.org/10.1016/j.bios.2006.11.009
Akinay Y, Kazici HC, Akkus IN, Salman F (2021) Synthesis of 3D Sn doped Sb2O3 catalysts with differentmorphologies and their effects on the electrocatalytic hydrogen evolution reaction in acidic medium. Ceram Int 47:29515–29524. https://doi.org/10.1016/j.ceramint.2021.07.278
Chemistry LibreTexts, Electrochemistry Tables, P1: Standard Reduction Potentials by Element. Available online: https://chem.libretexts.org/Ancillary_Materials/Reference/Reference_Tables/Electrochemistry_Tables/P1%3A_Standard_Reduction_Potentials_by_Element.
Schwarzenbach G, Flaschka H (1969) Complexometric titrations. Methuen, London
Wang X, Du Y, Yang H, Tian S, Ge Q, Huang S, Wang M (2021) Removal of chloride ions from acidic solution with antimony oxides. J Ind Eng Chem 93:170–175. https://doi.org/10.1016/j.jiec.2020.09.020
Vlasov YG, Borota VA, Ermolenko YE (2003) Low-selective GaAs and GaSb semiconductor sensors for potentiometric analysis of liquid media. Russ J Appl Chem 76:569–571. https://doi.org/10.1023/A:1025726901649
Burakhta VA, Sataeva SS (2014) Electrochemically modified semiconductor gallium arsenide electrodes for the argentometric titration of chlorides with silver nitrate in natural samples. J Analyt Chem 69:1079–1082. https://doi.org/10.1134/S1061934814110033
Acknowledgements
The obtaining of PEO layers, their study by XRD, and EDX methods were performed within the framework of the Institute of Chemistry FEB RAS State Order (project no. FWFN(0205)-2022-0001). Electroanalytical properties of the composites were studied at the Far Eastern Federal University.
Funding
Far East Branch, Russian Academy of Sciences, FWFN(0205)-2022-0001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vasilyeva, M.S., Lukiyanchuk, I.V., Shchitovskaya, E.V. et al. Multifunctional sensors based on TiO2-Sb-SbOx films, formed by anodic–cathodic electrochemical treatment of titanium. J Appl Electrochem 52, 1747–1760 (2022). https://doi.org/10.1007/s10800-022-01745-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01745-3