Abstract
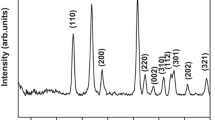
Under ultra violet visible light radiation, the photocatalytic degradation behaviour of different dyes such as Methylene Blue(MB), Methyl Orange(MO), Rhodamine B(Rh.B), and textile dye catalysed by tin oxide nanoparticles was investigated. Tin oxide nano powders were meticulously produced using the sol–gel acrylamide method. The manufactured powder was heated at various temperatures of 300 °C, 400 °C, 500 °C and 600 °C. After the heat treatment the powder were analysed by various techniques such as X-Ray Diffraction (XRD) techniques, Transmission Electron Microscopy (TEM), Raman spectroscopy, UV–Visible diffuse reflectance spectra and photocatalytic activity studies. All of the powder samples exhibit a tetragonal rutile structure of tin oxide, and XRD measurements were used to determine the grain size of the nanoparticles, which ranged from 7 to 18 nm. Surface morphology and microstructure characteristics that are almost spherical in the form its particle size was 20 nm observed in TEM. Raman spectra indicate three basic Raman peaks at 481, 632 and 773 cm−1 correspond to the Eg, A1g, and B2g vibration modes. The UV–Visible diffuse reflection spectra (330 nm and 450 nm) were helpful to identify the band gap of tin oxide its value in the range of 4.15 eV to 3.92 eV and also it is suitable materials for photocatalytic irradiation. Photocatalytic activity of tin oxide nanoparticles behaviour compared with four dyes using UV–Visible light radiation. From the observation to measure the pseudo-first-order rate constants of four dyes were computed. The removal efficiency of various dye was observed which indicate MB has greater efficiency (93%) it is higher than all other dyes. Within 90 min, 30 mg/L tin oxide at pH 11 caused the most deterioration. These findings point to the role of photogenerated holes in the dye's degradation process.
Graphical abstract









Similar content being viewed by others
References
Deshmukh SB, Bari RH, Patil GE, Kajale DD, Jain GH, Patil LA (2012) Preparation and characterization of zirconia based thick film resistor as a ammonia gas sensor. Int J Smart Sens Intell Syst 5(3):540–558. https://doi.org/10.21307/IJSSIS-2017-494
Chappel S, Zaban A (2002) Nanoporous SnO2 for dye sensitized solar cell improved cell performance by the synthesis of 18 nm SnO2 colloids. Sol Energy Mater Sol Cells 71:141–152. https://doi.org/10.1016/S0927-0248(01)00050-2
Gnanam S, Rajendran V (2010) Luminance properties of EG assisted SnO2 nano particles by sol–gel process. Dig J Nanomater Biostruct 5:699–704. https://doi.org/10.1016/S0927-0248(01)00050-2
Pouretedal HR, Kadkhodaie A (2010) Synthetic CeO2 nano particle catalysis of methylene blue photodegradation: kinetics and mechanism. Chin J Catal 31:1328–1334. https://doi.org/10.1016/s1872-2067(10)60121-0
Pouretedal HR, Kadkhodaie A (2010) Synthetic CeO2 nano particle catalysis of methylene blue photodegradation: kinetics and mechanism”. Chin J Catal 31:1328–1334
Jiang H, Wang Q, Zang S, Li J, Wang X (2014) Hydrothermal synthesis of high efficiency Pr, N, P tridoped TiO2 from TiCl4 hydrolysis and mechanism of its enhanced photoactivity. J Alloys Compd 600:34–42. https://doi.org/10.1016/j.jallcom.2014.02.083
Hernandez-Alonse MD, Fresno F, Suarez S, Coronado JM (2009) Development of alternative photocatalysts to TiO2: challenges and opportunities. Energy Environ Sci 2:1231–1257. https://doi.org/10.1039/B907933E
Tan G, Zhang L, Ren H, Wei S, Huang J, Xia A (2013) Effects of pH on the hierarchical structures and photocatalytic performance of BiVO4 powders prepared via microwave hydrothermal method. ACS Appl Mater Interfaces 5:5186–5193. https://doi.org/10.1021/am401019m
Peng S, Huang Y, Li Y (2013) Rare each doped TiO2–CdS and TiO2–CdS composites with improvement of photocatalytic hydrogen evolution under visible light irradiation. Mater Sci Semicond Process 16:62–69. https://doi.org/10.1016/j.msssp.2012.06.019
Matei Ghimbeu C, Van Landschoot RC, Schoonman J, Lumbreras M (2007) Preparation and characterization of SnO2 and Cu-doped SnO2 thin films using electrostatic spray deposition (ESD). J Eur Ceram Soc 27:207. https://doi.org/10.1016/j-jeurceramsoc.2006.05.092
Khandelwal R, Singh AP, Kapoor A, Grigorescu S, Miglietta P, Stankova NE, Perrone A (2009) Effect of deposition temperature on the structural and morphological properties of SnO2 films fabricated by pulse laser deposition. Opt Laser Technol 41:89. https://doi.org/10.1016/j-optlastec.2008.03.010
Zhao S, Zhou Y, Wang S, Zhao K, Han P (2006) Effect of ambient oxygen pressure on structural, optical and electrical properties of SnO2 thin films. Rare Met 25:693. https://doi.org/10.1016/S1001-0521(07)60014-X
Kwoka M, Ottaviano L, Passacantando M, Czempik G, Santucci S, Szuber J (2008) XPS study of the surface chemistry of Ag covered L-CVD SnO2 thin films. Appl Surf Sci 254:8089. https://doi.org/10.1016/j.apsusc.2008.03.019
Kong J, Deng H, Yang P, Chu J (2009) Synthesis and properties of pure and antimony doped tin dioxide thin films fabricated by sol–gel technique on silicon wafer. Mater Chem Phys 114:854. https://doi.org/10.1016/j-matchemphys.2008-10.049
Cao X, Cao L, Yao W, Ye X (1998) Influences of dopants on the electronic structure of SnO2 thin films. Thin Solid Films 317:443. https://doi.org/10.1016/S0040-6090(97)00638-x
Phillips HM, Li Y, Bi Z, Zhang B (1996) Reactive pulse laser deposition and laser induced crystallization of SnO2 transparent conducting thin films. Appl Phys A 63:347. https://doi.org/10.1016/BF01567325
Godbole VP, Vispute RD, Chaudhari SM, Kanetkar SM, Ogale SB (1990) Dependence of the properties of laser deposited tin oxide films on process variable. Mater Res 5:372. https://doi.org/10.1557/JMR1990.0372
Dolbec R, El Khakani MA, Serventi AM, Trudeau M, Saint-Jacques RG (2002) Microstructure and physical properties of nano structured tin oxide thin films grown by means of pulsed laser deposition. Thin Solid Films 419:230. https://doi.org/10.1016/S0040-6090(02)00769-1
Najjar M, Hosseini HA, Masoudi A, Sabouri Z, Mostafapour A, Khatami M, Darroudi M (2021) Green chemical approach for the synthesis of SnO2 nanoparticles and its application in photocatalytic degradation of Eriochrome Black T dye. Optik 242:167152. https://doi.org/10.1016/j.ijleo.2021.167152
Ebrahimian J, Mohsennia M, Khayatkashani M (2020) Photocatalytic-degradation of organic dye and removal of heavy metal ions using synthesized SnO2 nanoparticles by Vitex agnus-castus fruit via a green route. Mater Lett 263:127255. https://doi.org/10.1016/j.matlet.2019.127255
Honarmand M, Golmohammadi M, Naeimi A (2019) Biosynthesis of tin oxide (SnO2) nanoparticles using jujube fruit for photocatalytic degradation of organic dyes. Adv Powder Technol 30(8):1551–1557. https://doi.org/10.1016/j.apt.2019.04.033
Luque PA, Garrafa-Gálvez HE, Nava O, Olivas A, Martínez-Rosas ME, Vilchis-Nestor AR, Villegas-Fuentes A, Chinchillas-Chinchillas MJ (2021) Efficient sunlight and UV photocatalytic degradation of Methyl Orange, Methylene Blue and Rhodamine B, using Citrus × paradisi synthesized SnO2 semiconductor nanoparticles. Ceram Int 47(17):23861–23874. https://doi.org/10.1016/j.ceramint.2021.05.094
Fathima Beevi A, Sreekala G, Beena B (2021) Synthesis, characterization and photocatalytic activity of SnO2, ZnO nanoparticles against Congo red: a comparative study. Mater Today Proc 45(4):4045–4051. https://doi.org/10.1016/j.matpr.2020.10.755
Fu Z, Qin B, Guo X, Wang Y, Xu Y, Qiao Q, Wang Q, Wang F, Gao S, Yang Z (2021) Enhancement of photocatalytic dye degradation and photoconversion capacity of graphene oxide/SnO2 nanocomposites. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2021.162796 (Available online 25 Nov 2021)
Begum S, Ahmaruzzaman Md (2018) Green synthesis of SnO2 nanoparticles loaded on activated carbon and its application as photocatalyst in the degradation of alizarin red S dye. Mater Today Proc 5(1, Part 2):2314–2320. https://doi.org/10.1016/j.matpr.2017.09.235
Kar A, Olszówka J, Sain S, Sloman S-RI, Montes O, Fernández A, Pradhan SK, Wheatley AEH (2019) Morphological effects on the photocatalytic properties of SnO2 nanostructures. J Alloys Compd 810:151718. https://doi.org/10.1016/j.jallcom.2019.151718
Ahmed A, Siddique MN, Alam U, Ali T, Tripathi P (2019) Improved photocatalytic activity of Sr doped SnO2 nanoparticles: a role of oxygen vacancy. Appl Surf Sci 463:976–985. https://doi.org/10.1016/j.apsusc.2018.08.182
Harish S, Sabarinathan M, Archana J, Navaneethan M, Nisha KD, Ponnusamy S, Gupta V, Muthamizhchelvan C, Aswal DK, Ikeda H, Hayakawa Y (2017) Synthesis of ZnO/SrO nanocomposites for enhanced photocatalytic activity under visible light irradiation. Appl Surf Sci 418:147–155. https://doi.org/10.1016/j.apsusc.2017.01.164
Harish S, Archana J, Sabarinathan M, Navaneethan M, Nisha KD, Ponnusamy S, Gupta V, Muthamizhchelvan C, Aswal DK, Ikeda H, Hayakawa Y (2017) Controlled structural and compositional characteristic of visible light active ZnO/CuO photocatalyst for the degradation of organic pollutant. Appl Surf Sci 418:103–112. https://doi.org/10.1016/j.apsusc.2016.12.082
Srinivasan N, Anbuchezhiyan M, Harish S, Ponnusamy S (2019) Hydrothermal synthesis of C doped ZnO nanoparticles coupled with BiVO4 and their photocatalytic performance under the visible light radiation. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2019.07.093
Yin J, Huang S, Jian Z, Wang Z, Zhang Y (2015) “Fabrication of heterojunction SnO2/BiVO4 composites having enhanced visible light photocatalystic activity. Mater Sci Semicond Process 34:198–204. https://doi.org/10.1016/j.mssp.2015.02.044
Wang W, Xu C, Wang G, Liu Y, Zheng C (2002) Synthesis and Raman scattering study of rutile SnO2 nanowires. J Appl Phys 92:2740. https://doi.org/10.1016/1.1497718
Abello L, Bochu B, Gaskv A, Koudryavtseva S, Lucazeau G, Roumyantesva M (1998) Structural characterization of nanocrystalline SnO2 by X-ray and Raman spectroscopy. J Solid State Chem 135:78
Ramanathan G, John Xavier R, Murali KR (2012) Sol gel dip coated tin oxide thin films. Elixir Thin Film Technol 50:10588–10590
He Z, Shi Y, Gao C, Wen L, Chen J, Song S (2014) BiOCl/BiVO4 p–n heterojunction with enhanced photocatalytic activity under visible light irradiation. J Phys Chem C 118:389–398. https://doi.org/10.1021/jp409598s
Kuriakose S, Bhardwaj N, Singh J, Satpati B, Mohapatra S (2013) Structural, optical and photocatalytic properties of flower-like ZnO nano structures prepared by a facile wet chemical method. Beilstein J Nanotechnol 4:763–770. https://doi.org/10.3762/bjnano.4.87
Martinez A, Perez UMG (2010) Photocatalytic properties of BiVO4 prepared by the co-precipitation method degradation of RB and possible reaction mechanism under visible irradiation. Mater Res Bull 45:135–141. https://doi.org/10.1016/j.materresbull.2009.09.029
Suwanechawalit C, Buddee S, Wangnawa S (2017) Triton X-100 induced cuboid like BiVO4 microsphere with high photocatalytic performance. J Environ Sci 55:257–265. https://doi.org/10.1016/j.jes.2016-04.030
Baig A, Rathinam V, Palaninathan J (2020) Photodegradation activity of yttrium-doped SnO2 nanoparticles against methylene blue dye and antibacterial effects. Appl Water Sci 10(2):13. https://doi.org/10.1007/s13201-020-1143-1
Sivasankar Koppala, Ramdas Balan, Indranil Banerjee, Kangqiang Li, Lei Xu, Hua Liu, D.Kishore Kumar, Kakarla Raghava Reddy, veera sadhu (2021) Room temperature synthesis of novel worm like tin oxide nanoparticles for photocatalytic degradation of organic pollutants. Mater Sci Energy Technol 4:113–118. https://doi.org/10.1016/j.mset.2021.03.002
Ghaderi A, Abbas S, Farahbod F (2015) Synthesis of SnO2 and ZnO Nanoparticles and SnO2–ZnO hybrid for the photocatalytic oxidation of methyl orange. Iran J Chem Eng 12:96–105
Kusdianto K, Hudandini M, Kusuma TC, Widiyastuti W, Madhania S, Machmudah S, Nurtono T, Puspitasari D, Winar S (2020) Photocatalytic degradation of organic waste derived from textile dye by ZnO–Ag nanocomposite synthesized by spray pyrolysis. AIP Conf Proc 2219:030001. https://doi.org/10.1063/5.0003220
Ramanathan G , Murali K R (2018) Photocatalytic activity of biosynthesized CeO2 nano particles. SN Appl Sci. https://doi.org/10.1007/s42452-018-0103-Y
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramanathan, G., Murali, K.R. Photocatalytic activity of SnO2 nanoparticles. J Appl Electrochem 52, 849–859 (2022). https://doi.org/10.1007/s10800-022-01676-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01676-z