Abstract
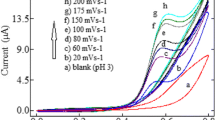
A novel electrochemical sensor containing an ionic liquid carbon paste electrode was synthesized to determine carbendazim in grape samples. The 2-hydroxy ethylammonium acetate (2-HEAA) was used as a modifier in the paste for electrode fabrication (2-HEAA-CPE), conferring higher detectability of the analyte compared to the unmodified electrode. The electrode was characterized by cyclic voltammetry in which the carbon paste electrode containing 10% of 2-hydroxy ethylammonium acetate (2-HEAA) was selected for the determination of carbendazim by Adsorptive Differential Pulse Stripping Voltammetry (AdDPSV). In optimal conditions for AdDPSV of carbendazim, the Dynamic Linear Response (DLR) of the method ranged from 0.009 to 0.476 µmol L−1, whereas the limits of detection and quantification were 1.69 nmol L−1 and 5.63 nmol L−1, respectively. The repeatability and reproducibility of the method produced relative standard deviations of 5.96% and 5.63%, respectively, and the recovery ranged from 89.0 to 100.6%, indicating the method can be successfully applied to determine CBZ in wine, grape mash, and grape juice. The synthesis of the electrode only takes a few steps and requires a simple pretreatment, making it a promising alternative to detecting pesticide residues in food samples.
Graphical abstract
The modified PIL electrode containing 2-hydroxy ethylammonium acetate (2-HEAA), which requires a fast and low-cost synthesis, showed good results for the determination of the carbendazim at small concentrations (nmol L-1) in industrialized wine, grape mash, and juice samples











Similar content being viewed by others
References
Hayes R, Warr GG, Atkin R (2015) Structure and nanostructure in ionic liquids. Chem Rev 115(13):6357–6426. https://doi.org/10.1021/cr500411q
Greaves TL, Drummond CJ (2015) Protic ionic liquids: evolving structure-property relationships and expanding applications. Chem Rev 115(20):11379–11448. https://doi.org/10.1021/acs.chemrev.5b00158
Xue Z, Qin L, Jiang J, Mu T, Gao G (2018) Thermal, electrochemical and radiolytic stabilities of ionic liquids. Phys Chem Chem Phys 20:8382–8402. https://doi.org/10.1039/C7CP07483B
Miran MS, Hoque M, Yasuda T et al (2019) Key factor governing the physicochemical properties and extent of proton transfer in protic ionic liquids: ΔpKa or chemical structure. Phys Chem Chem Phys 21:418–426. https://doi.org/10.1039/C8CP06973E
Rodrigues RDP, Castro FC, Santiago-Aguiar RS, Rocha MVP (2018) Ultrasound-assisted extraction of phycobiliproteins from Spirulina (Arthrospira) platensis using protic ionic liquids as solvent. Algal Res 31:454–462. https://doi.org/10.1016/j.algal.2018.02.021
Rodrigues RDP, Lima PF, Santiago-Aguiar RS, Rocha MVP (2019) Evaluation of protic ionic liquids as potential solvents for the heating extraction of phycobiliproteins from Spirulina (Arthrospira) platensis. Algal Res 38:101391. https://doi.org/10.1016/j.algal.2018.101391
Oliveira NS, Carlos ALS, Lima AS et al (2018) Ionic liquid-based ultrasonic-assisted extraction of alkaloids from cacao (theobroma cacao). Chem Eng Trans 64:49–54. https://doi.org/10.3303/CET1864009
Dias RM, Sosa FHB, Costa MC (2020) Dissolution of lignocellulosic biopolymers in ethanolamine-based protic ionic liquids. Polym Bull 77(7):3637–3656. https://doi.org/10.1007/s00289-019-02929-2
Rocha EGA, Pin TC, Rabelo SC, Costa AC (2017) Evaluation of the use of protic ionic liquids on biomass fractionation. Fuel 206:145–154. https://doi.org/10.1016/j.fuel.2017.06.014
Boli E, Dimou E, Voutsas E (2018) Separation of the isopropanol-water azeotropic mixture using ionic liquids. Fluid Ph Equilib 456:77–83. https://doi.org/10.1016/j.fluid.2017.10.003
Santos MO, Santos GOS, Mattedi S, Griza S, Eguiluz KIB, Salazar-Banda GR (2018) Influence of the calcination temperature and ionic liquid used during synthesis procedure on the physical and electrochemical properties of Ti/(RuO2)0.8-(Sb2O4)0.2 anodes. J Electroanal Chem 829:116–128. https://doi.org/10.1016/j.jelechem.2018.10.013
Sobhani S, Nasseri F, Zarifi F (2018) Unique role of 2-hydroxy ethylammonium acetate as an ionic liquid in the synthesis of Fe3O4 magnetic nanoparticles and preparation of pyridine derivatives in the presence of a new magnetically recyclable heterogeneous catalyst. J Iran Chem Soc 15:2721–2732. https://doi.org/10.1007/s13738-018-1460-6
Shi X, Ding M, Li C, Wang W, Guo H (2018) A green synthesis of highly functionalized 3-amino-2-phenylsulfonyl-1-alkyl/aryl-1H-pyrazolo[1,2-b]phthalazine-5,10-diones and their reduction and photophysical studies. J Heterocycl Chem 55:440–446. https://doi.org/10.1002/jhet.3061
MacFarlane DR, Tachikawa N, Forsyth M et al (2014) Energy applications of ionic liquids. Energy Environ Sci 7:232–250. https://doi.org/10.1039/C3EE42099J
Đorđević JS, Maksimović VM, Gadžurić SB, Trtić-Petrović TM (2017) Determination of carbendazim by an ionic liquid-modified carbon paste electrode. Anal Lett 50:1075–1090. https://doi.org/10.1080/00032719.2016.1210615
Shiddiky MJA, Torriero AAJ (2011) Application of ionic liquids in electrochemical sensing systems. Biosens Bioelectron 26(5):1775–178717. https://doi.org/10.1016/j.bios.2010.08.064
Opallo M, Lesniewski A (2011) A review on electrodes modified with ionic liquids. J Electroanal Chem 656(1–2):2–16. https://doi.org/10.1016/j.jelechem.2011.01.008
Abo-Hamad A, Al Saadi MA, Hayyan M, Juneidi I, Hashim MA (2016) Ionic liquid-carbon nanomaterial hybrids for electrochemical sensor applications: a review. Electrochim Acta 193:321–343. https://doi.org/10.1016/j.electacta.2016.02.044
Maximiano EM, Cardoso CAL, Arruda GJ (2016) Detecção eletroquímica de carbendazim em sucos de frutas cítricas utilizando eletrodo de pasta de carbono. J. Orbital Electron J Chem 8(4):232–239. https://doi.org/10.17807/orbital.v8i4.797
Silva RC, Barros K, Pavão AC (2014) Carcinogenicidade do carbendazim e seus metabólitos. Quim Nova 37(8):1329–1334. https://doi.org/10.5935/0100-4042.20140214
Global® Crop Protetion (2020) https://globalcropprotection.com/2018/04/12/brasil-importa-4-5-mil-toneladas-de-carbendazim-em-2017/. Accessed 14 January 2020
Ministério da Saúde, Agência Nacional de Vigilância Sanitária (ANVISA) (2019) http://portal.anvisa.gov.br/. Accessed 03 February 2020
Lee HS, Rahmana MdM, Chung HS et al (2018) An effective methodology for simultaneous quantification of thiophanate-methyl, and its metabolite carbendazim in pear, using LC-MS/MS. J Chromatogr B 1095(1–7):1570–2232. https://doi.org/10.1016/j.jchromb.2018.07.010
Wang X, Jia R, Song Y et al (2019) Determination of pesticides and their degradation products in water samples by solid-phase extraction coupled with liquid chromatography-mass spectrometry. Microchem J 149:104013. https://doi.org/10.1016/j.microc.2019.104013
Maximiano EM, Lima F, Cardoso CAL, Arruda GJ (2018) Modification of carbon paste electrodes with recrystallized zeolite for simultaneous quantification of thiram and carbendazim in food samples and an agricultural formulation. Electrochim Acta 259:66–76. https://doi.org/10.1016/j.electacta.2017.10.162
Bakirhan NK, Uslu B, Ozkan SA (2018) Chapter 5–the detection of pesticide in foods using electrochemical sensors. In: Grumezescu AM, Holban AM (eds) Food Safety and Preservation. Academic Press, London, pp 91–141
Weia P, Gan T, Wu K (2018) N-methyl-2-pyrrolidone exfoliated graphene as highly sensitive analytical platform for carbendazim. Sens Actuators B 274:551–559. https://doi.org/10.1016/j.snb.2018.07.174
Liao X, Huang Z, Huang K et al (2019) Highly sensitive detection of carbendazim and its electrochemical oxidation mechanism at a nanohybrid sensor. J Electrochem Soc 166:B322–B327. https://doi.org/10.1149/2.0251906jes
Santana PCA, Lima JBS, Sussuchi EM et al (2019) Semiconductor nanocrystals-reduced graphene composites for the electrochemical detection of carbendazim. J Braz Chem Soc 30(6):1302–1308. https://doi.org/10.21577/0103-5053.20190026
Tian C, Cui R, Zhang G et al (2019) Three-dimensional nanoporous copper and reduced graphene oxide composites as enhanced sensing platform for electrochemical detection of carbendazim. J Electroanal Chem 847:113243. https://doi.org/10.1016/j.jelechem.2019.113243
Alvarez VH, Mattedi S, Martin-Pastor M, Aznar M, Iglesias M (2011) Thermophysical properties of binary mixtures of {ionic liquid 2-hydroxy ethylammonium acetate + (water, methanol, or ethanol). J Chem Thermodyn 43:997–1010. https://doi.org/10.1016/j.jct.2011.01.014
Oliveira LH, Souza ACD, Pizzuti L et al (2014) Determinação Voltamétrica do Antioxidante Galato de Propila em Biodiesel Empregando Eletrodos de Pasta de Carbono Modificados com Líquido Iônico. Orbital Electron J Chem 6(4):255–266. https://doi.org/10.17807/orbital.v6i4.635
Sant’Anna MVS, Carvalho SWMM, Sussuchi EM, et al (2020) Electrochemical sensor based on biochar and reduced graphene oxide nanocomposite for carbendazim determination. Talanta 220(1–8):121334. https://doi.org/10.1016/j.talanta.2020.121334
Barbosa LCA (2013) Espectroscopia No Infravermelho Na Caracterização de Compostos Orgânicos, 1ª ed.; Editora UFV.
Schmitzhaus TE, Vega MRO, Schroeder R et al (2020) An amino-based protic ionic liquid as a corrosion inhibitor of mild steel in aqueous chloride solutions. Mater Corros 71(7):1175–1193. https://doi.org/10.1002/maco.201911347
Camargo D, Andrade RS, Iglesias M et al (2016) Investigation of the rheological properties of protic ionic liquids. J Phys Org Chem 29(11):604–612. https://doi.org/10.1002/poc.3553
Milhome MAL, Sousa DOB, Lima FAF, Nascimento RF (2009) Avaliação do potencial de contaminação de águas superficiais e subterrâneas por pesticidas aplicados na agricultura do Baixo Jaguaribe. CE Eng Sanit Ambient 14(3):363–372. https://doi.org/10.1590/S1413-41522009000300010
Chemicalize. Calculate properties, search chemical data, and draw molecules online. chemicalize.com/#/calculation. Accessed 24 November 2019.
Brett AMO, Brett CMA (1996) Eletroquímica: Princípios, Métodos e Aplicações. Oxford University Press, Oxford
Pacheco WF, Semaan FS, Almeida VGK, Ritta AGSL, Aucélio RQ (2013) Voltametrias: Uma Breve Revisão Sobre os Conceitos. Rev Virtual Quim 5:516–537. https://doi.org/10.5935/1984-6835.20130040
Li K, Li Y, Wang L, Lingxi Y, Ye B (2019) Study the voltammetric behavior of 10-Hydroxycamptothecin and its sensitive determination at electrochemically reduced graphene oxide modified glassy carbon electrode. Arab J Chem 12(8):2732–2739. https://doi.org/10.1016/j.arabjc.2015.05.014
Fotouhi L, Hashkavayi AB, Heravi MM (2013) Electrochemical behaviour and voltammetric determination of sulphadiazine using a multi-walled carbon nanotube composite film-glassy carbon electrode. J Exp Nanosci 8(7–8):947–956. https://doi.org/10.1080/17458080.2011.624554
Siswana M, Ozoemena KI, Nyokong T (2008) Electrocatalytic Detection of amitrole on the multi-walled carbon nanotube—iron (II) tetra-aminophthalocyanine Platform. Sensors 8:5096–5105. https://doi.org/10.3390/s8085096
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem Interf Electrochem 101(1):19–28. https://doi.org/10.1016/S0022-0728(79)80075-3
Nurzulaikha R, Lim HN, Harrison I et al (2015) Graphene/SnO2 nanocomposite-modified electrode for electrochemical detection of dopamine. Sens Bio-Sens Res 5(42–49):2214–1804. https://doi.org/10.1016/j.sbsr.2015.06.002
Saleh GA, Askal HF, Refaat IH, Naggar AH, Abdel-aal FAM (2016) Adsorptive square wave voltammetric determination of the antiviral drug valacyclovir on a novel sensor of copper microparticles–modified pencil graphite electrode. Arab J Chem 9(1):143–151. https://doi.org/10.1016/j.arabjc.2015.08.015
Luo S, Wu Y, Gou H (2013) A voltammetric sensor based on GO–MWNTs hybridnanomaterial-modified electrode for determination of carbendazim in soil and water samples. Ionics 19:673–680. https://doi.org/10.1007/s11581-013-0868-3
Gao X, Gao Y, Bian C, Ma H, Liu H (2019) Electroactive nanoporous gold driven electrochemical sensor for the simultaneous detection of carbendazim and methyl parathion. Electrochim Acta 310(78–85):0013–4686. https://doi.org/10.1016/j.electacta.2019.04.120
Acknowledgements
The authors thank the Brazilian development agencies CNPq and CAPES for their financial support, the Corrosion and Nanotechnology Laboratory—LCNT, the Sergipe Oil and Gas Competence Center—NUPEG/PETROBRAS/UFS, the Multiuser Chemistry Laboratory Center—CLQM, the Graduate Program in Chemistry (PPGQ) of the Federal University of Sergipe for the infrastructure support, and to Laboratory of Nuclear Magnetic Resonance—LabRMN, Institute of Chemistry, Federal University of Goiás, for supporting NMR analyses.
Author information
Authors and Affiliations
Contributions
JFM: Conceptualization, Validation, Formal analysis, Investigation, Visualization, Writing—original draft, and Methodology. AACA: Writing—original draft and Methodology. MVSS: Conceptualization, Validation, Visualization, Writing—original draft and Methodology. FGCC: Conceptualization, Resources, Visualization, Writing—review & editing, and Project administration and revision. GARO: Structural characterization by NMR and revision. LML: Structural characterization by NMR and revision. EMS: Conceptualization, Methodology, Supervision, Resources, Visualization, Funding acquisition, Writing—review & editing, and Project administration and revision.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10800_2021_1665_MOESM1_ESM.docx
All the information related to the distribution of carbendazim species at different pH and the effect of organic and inorganic on the analytical signal of CBZ at low concentrations, as well as the relative standard deviations of the electrode are shown in the supplementary information section. Supplementary file1 (DOCX 475 kb)
Rights and permissions
About this article
Cite this article
de Macedo, J.F., Alves, A.A.C., Sant’Anna, M.V.S. et al. Electrochemical determination of carbendazim in grapes and their derivatives by an ionic liquid-modified carbon paste electrode. J Appl Electrochem 52, 729–742 (2022). https://doi.org/10.1007/s10800-021-01665-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-021-01665-8