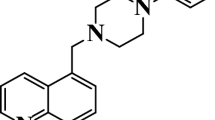
Abstract
The preventing effect of novel triphenyl imidazole based on 8-hydroxyquinoline (HDiPIQ) on the corrosion attack of mild steel in 1 M HCl solution was investigated. Electrochemical impedance and potentiodynamic polarization measurements have been realized. Inhibitor concentration increase leads to a considerable shift in mild steel oxidization rate in molar hydrochloric acid solution with inhibitive efficiency such as 89% at 10–3 M concentration of HDiPIQ. Tafel curves showed that HDiPIQ acted as a mixed-type inhibitor. Nyquist curve drawing showed that inhibitor concentration getting increased resulted in polarization resistance growth, that involves inhibition efficiency increase and double-layer capacitance decrease. The effect of temperature in the range 298–328 K at a concentration of 10–3 M HDiPIQ showed that inhibiting efficiency diminished slightly with temperature. HDiPIQ adsorption on steel surface has been found to comply with Langmuir’s isotherm model, implying a mixed type of binding with predominance of chemical adsorption mechanism. Activation energy and thermodynamic results such as enthalpy, entropy and free energy have been determined and discussed. Surface characterization using scanning electron microscopy allowed the confirmation of HDiPIQ inhibiting effect on mild steel corrosion. UV–visible spectroscopy results have been analyzed and discussed. Quantum chemical factors such as the frontier molecular orbitals energies (EHOMO and ELUMO), the energy gap between ELUMO and EHOMO (ΔE), hardness (η), electronegativity (χ), and Fukui indexes have been calculated and discussed using density functional theory calculations and the Becke’s three-parameter exchange functional with the Lee–Yang–Parr correlation function and the 6–311 + G(d,p) basis set. Monte Carlo simulation allowed elucidating inhibitor/mild steel interaction of the above-mentioned inhibitor molecule.
Graphic abstract
















Similar content being viewed by others
References
Rbaa M, Benhiba F, Obot IB, Oudda H, Warad I, Lakhrissi B, Zarrouk A (2019) Two new 8-hydroxyquinoline derivatives as an efficient corrosion inhibitors for mild steel in hydrochloric acid: synthesis, electrochemical, surface morphological, UV–visible and theoretical studies. J Mol Liq 276:120–133
El Faydy M, Lakhrissi B, Guenbour A, Kaya S, Bentiss F, Warad I, Zarrouk A (2019) In situ synthesis, electrochemical, surface morphological, UV–visible, DFT and Monte Carlo simulations of novel 5-substituted-8-hydroxyquinoline for corrosion protection of carbon steel in a hydrochloric acid solution. J Mol Liq 280:341–359
El Faydy M, Touir R, Touhami ME, Zarrouk A, Jama C, Lakhrissi B, Olasunkanmi EEE, Bentiss F (2018) Corrosion inhibition performance of newly synthesized 5-alkoxymethyl-8-hydroxyquinoline derivatives for carbon steel in 1 M HCl solution: experimental, DFT and Monte Carlo simulation studies. Phys Chem Chem Phys 20:20167–20187
Laabaissi T, Lgaz H, Oudda H, Benhiba F, Zarrok H, Zarrouk A, El Midaoui A, Lakhrissi B, Touir R (2017) Comparative study of corrosion inhibition effect of benzodiazepine derivative on the carbon steel surface in 2.0 M H3PO4 and 1.0 M HCl mediums: electrochemical, theoretical and Monte Carlo simulations studies. J Mater Environ Sci 8:1054–1067
Verma C, Quraishi MA, Singh A (2016) A thermodynamical, electrochemical, theoretical and surface investigation of diheteroaryl thioethers as effective corrosion inhibitors for mild steel in 1 M HCl. J Taiwan Inst Chem Eng 58:127–140
Zarrok H, Zarrouk A, Salghi R, Oudda H, Hammouti B, Touhami ME, Bouachrine M, Boukhris S (2012) A combined experimental and theoretical study on the corrosion inhibition and adsorption behaviour of quinoxaline derivative during carbon steel corrosion in hydrochloric acid. Port Electrochim Acta 30:405–417
Obot IB, Onyeachu IB (2018) Electrochemical frequency modulation (EFM) technique: theory and recent practical applications in corrosion research. J Mol Liq 249:83–96
Akalezi CO, Enenebaku CK, Oguzie EE (2012) Application of aqueous extracts of coffee senna for control of mild steel corrosion in acidic environments. Int J Ind Chem 3:1–12. https://doi.org/10.1186/2228-5547-3-13
El Faydy M, Benhiba F, Lakhrissi B, Touhami ME, Warad I, Bentiss F, Zarrouk A (2019) The inhibitive impact of both kinds of 5-isothiocyanatomethyl-8-hydroxyquinoline derivatives on the corrosion of carbon steel in acidic electrolyte. J Mol Liq 295:111629
Rbaa M, Lakhrissi B (2019) Novel oxazole and imidazole based on 8-hydroxyquinoline as a corrosion inhibition of mild steel in HCl solution: insights from experimental and computational studies. Surf Interfaces 15:43–59
Khadraoui A, Khelifa A, Hadjmeliani M, Mehdaoui R, Hachama K, Tidu A, Azari Z, Obot IB, Zarrouk A (2016) Extraction, characterization and anti-corrosion activity of Mentha pulegium oil: weight loss, electrochemical, thermodynamic and surface studies. J Mol Liq 216:724–731
Nabah R, Benhiba F, Ramli Y, Ouakki M, Cherkaoui M, Oudda H, Warad I, Zarrouk A (2018) Corrosion inhibition study of 5, 5-diphenylimidazolidine-2, 4-dione for mild steel corrosion in 1 M HCl solution: experimental, theoretical computational and Monte Carlo simulations studies. Anal Bioanal Electrochem 10:1375–1398
Ammal PR, Prajila M, Joseph A (2018) Effect of substitution and temperature on the corrosion inhibition properties of benzimidazole bearing 1, 3, 4-oxadiazoles for mild steel in sulphuric acid: physicochemical and theoretical studies. J Environ Chem Eng 6:1072–1085
Chidiebere MA, Oguzie EE, Liu L, Li Y, Wang F (2015) Adsorption and corrosion inhibiting effect of ribo fl avin on Q235 mild steel corrosion in acidic environments. Mater Chem Phys 156:95–104
Haque J, Srivastava V, Verma C, Quraishi MA (2017) Experimental and quantum chemical analysis of 2-amino-3-((4-((S)-2-amino-2-carboxyethyl)-1H-imidazol-2-yl)thio) propionic acid as new and green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution. J Mol Liq 225:848–855
Yıldız R (2019) Adsorption and inhibition effect of 2,4-diamino-6-hydroxypyrimidine for mild steel corrosion in HCl medium: experimental and theoretical investigation. Ionics (Kiel) 25:859–870
Solomon MM, Umoren SA, Quraishi MA, Mazumder J (2019) Corrosion inhibition of N80 steel in simulated acidizing environment by N-(2-(2-pentadecyl-4,5-dihydro-1H-imidazol-1-YL) ethyl) palmitamide. J Mol Liq 273:476–487
Kaya S, Tüzün B, Kaya C, Obot IB (2016) Determination of corrosion inhibition effects of amino acids: quantum chemical and molecular dynamic simulation study. J Taiwan Inst Chem Eng 58:528–535
Ouakki M, Rbaa M, Galai M, Lakhrissi B, Rifi EH, Cherkaoui M (2018) Experimental and quantum chemical investigation of imidazole derivatives as corrosion inhibitors on mild steel in 1.0 M hydrochloric acid. J Bio Tribo Corrosion 4:35. https://doi.org/10.1007/s40735-018-0151-2
Smitha M, Mary YS, Hossain M, Resmi KS, Armaković S, Armaković SJ, Pavithran R, Nanda AK, Van Alsenoy C (2018) Two novel imidazole derivatives—combined experimental and computational study. J Mol Struct 1173:221–239
Pestov AV, Privar YO, Mekhaev AV, Fedorets AN, Ezhikova MA, Kodess MI, Bratskaya SY (2019) A new approach to the green synthesis of imidazole-containing polymer ligands and cryogels. Eur Polym J 115:356–363
Abdulazeez I, Zeino A, Kee CW, Al-Saadi AA, Khaled M, Wong MW, Al-Sunaidi AA (2019) Mechanistic studies of the influence of halogen substituents on the corrosion inhibitive efficiency of selected imidazole molecules: a synergistic computational and experimental approach. Appl Surf Sci 471:494–505
Zhang L, Ge Y, Wang QM, Zhou CH (2019) Identification of novel imidazole flavonoids as potent and selective inhibitors of protein tyrosine phosphatase. Bioorg Chem 88:102900
Lopes-de-Campos D, Nunes C, Sarmento B, Jakobtorweihen S, Reis S (2018) Metronidazole within phosphatidylcholine lipid membranes: new insights to improve the design of imidazole derivatives. Eur J Pharm Biopharm 129:204–214
Okano Y, Saito-Tarashima N, Kurosawa M, Iwabu A, Ota M, Watanabe T, Kato F, Hishiki T, Fujimuro M, Minakawa N (2019) Synthesis and biological evaluation of novel imidazole nucleosides as potential anti-dengue virus agents. Bioorg Med Chem 27:2181–2186
Zhang GA, Hou XM, Hou BS, Liu HF (2019) Benzimidazole derivatives as novel inhibitors for the corrosion of mild steel in acidic solution: experimental and theoretical studies. J Mol Liq 278:413–427
Zhang Z, Chen S, Li Y, Li S, Wang L (2009) A study of the inhibition of iron corrosion by imidazole and its derivatives self-assembled films. Corros Sci 51:291–300
Stupnišek-Lisac E, Gazivoda A, Madžarac M (2002) Evaluation of non-toxic corrosion inhibitors for copper in sulphuric acid. Electrochim Acta 47:4189–4194
Curkovic HO, Stupnisek-Lisac E, Takenouti H (2010) The influence of pH value on the efficiency of imidazole based corrosion inhibitors of copper. Corros Sci 52:398–405
Benali O, Larabi L, Traisnel M, Gengembre L, Harek Y (2007) Electrochemical, theoretical and XPS studies of 2-mercapto-1-methylimidazole adsorption on carbon steel in 1 M HClO4. Appl Surf Sci 253:6130–6139
Sastri VS, Packwood RH, Brown JR, Bednar JS, Galbraith LE, Moore VE (1989) Corrosion inhibition by some oxyanions in coal-water slurries. Br Corros J 24:30–35. https://doi.org/10.1179/000705989798270469
Sastri VS, Perumareddi JR (1997) Molecular orbital theoretical studies of some organic corrosion inhibitors. Corrosion 53:617–622
Rbaa M, Errahmany N, El Kacimi Y, Galai M, El Faydy M, Touhami ME, Lakhrissi Y, Lakhrissi B (2018) Chemical and electrochemical studies of novel quinazolinone derivatives based on 8-hydroxyquinoline as corrosion inhibitor for mild steel in 1.0 M HCl solution. Anal Bioanal Electrochem 10:1328–1354
Khattabi M, Benhiba F, Tabti S, Djedouani A, El Assyry A, Touzani R, Warad I, Oudda H, Zarrouk A (2019) Performance and computational studies of two soluble pyran derivatives as corrosion inhibitors for mild steel in HCl. J Mol Struct 1196:231–244
Benhiba F, Hsissou R, Benzekri Z, Belghiti ME, Lamhamdi A, Bellaouchou A, Guenbour A, Boukhris S, Oudda H, Warad I, Zarrouk A (2020) Nitro substituent effect on the electronic behavior and inhibitory performance of two quinoxaline derivatives in relation to the corrosion of mild steel in 1 M HCl. J Mol Liq 312:113367
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M (2009) GAUSSIAN 09 Révision. Gaussian Inc, Wallingford CT
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3093
Miertuš S, Scrocco E, Tomasi J (1981) Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem Phys 55:117–129
Miertus̃ S, Tomasi J (1982) Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem Phys 65:239–245
Pascual-ahuir JL, Silla E, Tuñon I (1994) GEPOL: an improved description of molecular surfaces. III. A new algorithm for the computation of a solvent-excluding surface. J Comput Chem 15:1127–1138
Colominas C, Luque FJ, Teixidó J, Orozco M (1999) Cavitation contribution to the free energy of solvation: comparison of different formalisms in the context of MST calculations. Chem Phys 240:253–264
Improta R, Barone V, Scalmani G, Frisch MJ (2006) A state-specific polarizable continuum model time dependent density functional theory method for excited state calculations in solution. J Chem Phys 125:054103
Improta R, Scalmani G, Frisch MJ, Barone V (2007) Toward effective and reliable fluorescence energies in solution by a new state specific polarizable continuum model time dependent density functional theory approach. J Chem Phys 127:074504
Koopmans T (1933) Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica 1:104–113
Pearson RG (1988) Absolute electronegativity and hardness: application to inorganic chemistry. Inorg Chem 27:734–740
Lukovits I, Kálmán E, Zucchi F (2001) Corrosion inhibitors: correlation between chemical structure and efficiency. Corrosion 57:3–8
Ghailane T, Balkhmima RA, Ghailane R, Souizi A, Touir R, Touhami ME, Marakchi K, Komiha N (2013) Experimental and theoretical studies for mild steel corrosion inhibition in 1M HCl by two new benzothiazine derivatives. Corros Sci 76:317–324
Fuentealba P, Pérez P, Contreras R (2000) On the condensed Fukui function. J Chem Phys 113:2544–2551
Dassault Systems Materials Studio Tutorials, BIOVIA Support, 5005 Wateridge Vista Drive, San Diego, CA 92121, USA (2017).
Abousalem AS, Ismail MA, Fouda AS (2018) A complementary experimental and in silico studies on the action of fluorophenyl-2,2′-bichalcophenes as ecofriendly corrosion inhibitors and biocide agents. J Mol Liq 276:255–274
Fouda AS, Ismail MA, Al-Khamri AA, Abousalem AS (2019) Experimental, quantum chemical and molecular simulation studies on the action of arylthiophene derivatives as acid corrosion inhibitors. J Mol Liq 290:111–178
Verma C, Quraishi MA, Obot IB, Ebenso EE (2019) Effect of substituent dependent molecular structure on anti-corrosive behavior of one-pot multicomponent synthesized pyrimido [2,1-B] benzothiazoles: computer modelling supported experimental studies. J Mol Liq 287:110972
Huang W, Hu L, Liu C, Pan J, Tian Y, Cao K (2018) Corrosion inhibition of carbon steel by Lepidine in HCl solution. Int J Electrochem Sci 13:11273–11285
Tazouti A, Galai M, Touir R, Touhami ME, Zarrouk A, Ramli Y, Saraçoğlu M, Kaya S, Kandemirli F, Kaya C (2016) Experimental and theoretical studies for mild steel corrosion inhibition in 1.0 M HCl by three new quinoxalinone derivatives. J Mol Liq 221:815–832
Benhiba F, Serrar H, Hsissou R, Guenbour A, Bellaouchou A, Tabyaoui M, Boukhris S, Oudda H, Warad I, Zarrouk A (2020) Tetrahydropyrimido-Triazepine derivatives as anti-corrosion additives for acid corrosion: chemical, electrochemical, surface and theoretical studies. Chem Phys Lett 743:137181
Solmaz R, Altunba E, Karda G (2011) Adsorption and corrosion inhibition effect of 2-((5-mercapto-1,3,4- thiadiazol-2-ylimino)methyl)phenol Schiff base on mild steel. Mater Chem Phys 125:796–801
Ouakki M, Galai M, Rbaa M, Abousalem AS, Lakhrissi B, Rifi EH, Cherkaoui M (2019) Quantum chemical and experimental evaluation of the inhibitory action of two imidazole derivatives on mild steel corrosion in sulphuric acid medium. Heliyon 5:e02759
Döner A, Kardas G (2011) N-Aminorhodanine as an effective corrosion inhibitor for mild steel in 0.5 M H2SO4. Corros Sci 53:4223–4232
El Aoufir Y, Sebhaoui J, Lgaz H, El Bakri Y, Zarrouk A, Bentiss F, Guenbour A, Essassi EM, Oudda H (2017) Corrosion inhibition of carbon steel in 1M HCl by 1,5-benzodiazepine derivative: experimental and molecular modeling studies. J Mater Environ Sci 8:2161–2173
Dutta A, Saha SK, Banerjee P, Sukul D (2015) Correlating electronic structure with corrosion inhibition potentiality of some bis-benzimidazole derivatives for mild steel in hydrochloric acid: combined experimental and theoretical studies. Corros Sci 98:541–550
el Faydy M, Rbaa M, Lakhrissi L, Lakhrissi B, Warad I, Obot IB, Zarrouk A (2019) Corrosion protection of carbon steel by two newly synthesized benzimidazol-2-ones substituted 8-hydroxyquinoline derivatives in 1 M HCl: experimental and theoretical study. Surf Interfaces 14:222–237
Lopez DA, Simison SN, de Sanchez SR (2003) The influence of steel microstructure on CO2 corrosion. EIS studies on the inhibition efficiency of benzimidazole. Electrochim Acta 48:845–854
Haque J, Verma C, Srivastava V, Quraishi MA, Ebenso EE (2018) Experimental and quantum chemical studies of functionalized tetrahydropyridines as corrosion inhibitors for mild steel in 1 M hydrochloric acid. Results Phys 9:1481–1493
Loto RT, Loto CA, Joseph O, Olanrewaju G (2016) Adsorption and corrosion inhibition properties of thiocarbanilide on the electrochemical behavior of high carbon steel in dilute acid solutions. Results Phys 6:305–314
El Faydy M, Galai M, Touhami ME, Obot IB, Lakhrissi B, Zarrouk A (2017) Anticorrosion potential of some 5-amino-8-hydroxyquinolines derivatives on carbon steel in hydrochloric acid solution Gravimetric, electrochemical, surface morphological, UV–visible, DFT and Monte Carlo simulations. J Mol Liq 248:1014–1027
Kokalj A (2010) Is the analysis of molecular electronic structure of corrosion inhibitors sufficient to predict the trend of their inhibition performance. Electrochim Acta 56:745–755
Bockris JOM, Reddy AK, Gamboa-Aldeco M (1998) Modern electrochemistry: an introduction to an interdisciplinary area. Plenum Press, New York
Ahamad I, Prasad R, Quraishi MA (2010) Thermodynamic, electrochemical and quantum chemical investigation of some Schiff bases as corrosion inhibitors for mild steel in hydrochloric acid solutions. Corros Sci 52:933–942
Riggs O, Hurd R (1967) Temperature coefficient of corrosion inhibition. Corrosion 23:252–260
Alaoui K, Touir R, Galai M, Serrar H, Ouakki M, Kaya S, Tüzün B, Boukhris S, Ebn Touhami M, El Kacimi Y (2018) Electrochemical and computational studies of some triazepine carboxylate compounds as acid corrosion inhibitors for mild steel. J Bio Tribo Corros. https://doi.org/10.1007/s40735-018-0154-z
Rbaa M, Errahmany N, El Kacimi Y, Galai M, El Faydy M, Lakhrissi Y, Touhami ME, Lakhrissi B (2018) Chemical and electrochemical studies of novel quinazolinone derivatives based on 8-hydroxyquinoline as corrosion inhibitor for mild steel in 1.0 M HCl solution. Anal Bioanal Electrochem 10:1328–1354
Idouhli R, Oukhrib A, Koumya Y, Abouelfida A, Benyaich A, Benharref A (2018) Inhibitory effect of Atlas cedar essential oil on the corrosion of steel in 1 m HCl. Corros Rev 36:373–384
Ali AI, Mahrous YS (2017) Corrosion inhibition of C-steel in acidic media from fruiting bodies of Melia azedarach L. extract and a synergistic Ni2+ additive. RSC Adv 7:23687–23698
Palaniappan N, Cole I, Caballero-Briones F, Manickam S, Thomas KRJ, Santos D (2020) Experimental and DFT studies on the ultrasonic energy-assisted extraction of the phytochemicals of: Catharanthus roseus as green corrosion inhibitors for mild steel in NaCl medium. RSC Adv 10:5399–5411
El Faydy M, About H, Benhiba F, Lakhrissi B, Guenbour A, Bentiss F, Warad I, Ebenso EE, Zarrouk A (2020) The inhibitory effect of two 5-alkylthio-8-hydroxyquinoline salts on steel C22E in a molar electrolyte of hydrochloric acid: experimental and theoretical studies. Surf Interfaces 20:100575
Koumya Y, Idouhli R, Khadiri M, Abouelfida A, Aityoub A, Benyaich A, Romane A (2019) Pitting corrosion and effect of Euphorbia echinus extract on the corrosion behavior of AISI 321 stainless steel in chlorinated acid. Corros Rev 37(3):259–271
Fleming I (2010) Molecular orbitals and organic chemical reactions- reference edition. Wiley. https://doi.org/10.1002/9780470689493
Gece G (2008) The use of quantum chemical methods in corrosion inhibitor studies. Corros Sci 50:2981–2992
Fouda AS, Ismail MA, Abou-Shahba RM, Husien WA, El-Habab ES, Abousalem AS (2020) Experimental and computational chemical studies on the cationic furanylnicotinamidines as novel corrosion inhibitors in aqueous solutions. Chin J Chem Eng 28:477–491
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oubaaqa, M., Rbaa, M., Ouakki, M. et al. Novel triphenyl imidazole based on 8-hydroxyquinoline as corrosion inhibitor for mild steel in molar hydrochloric acid: experimental and theoretical investigations. J Appl Electrochem 52, 413–433 (2022). https://doi.org/10.1007/s10800-021-01632-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-021-01632-3