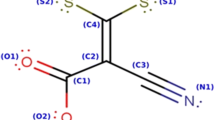
Abstract
In this work the adsorption and corrosion inhibition conduct of chalcone oxime derivatives on carbon steel in 0.5 M sulfuric acid solution at various temperatures (293, 303, 313, and 323 K) were investigated through weight loss measurements, potentiodynamic polarization, and electrochemical impedance spectroscopy. Results reveal that CO–H and CO–OMe exhibit an excellent inhibition efficiency of 95 and 96% at a concentration of 4.48 × 10−4 M and 293 K, respectively. The compounds are classified as mixed type inhibitors. Moreover, the influence of temperature and the activation parameters disclose that CO–H and CO–OMe are chemisorbed on the carbon steel surface. The adsorption of CO–H and CO–OMe follows Langmuir isotherm. The surface morphology was evaluated using scanning electron microscopy (SEM) and the adsorption behavior was analyzed by UV–visible. MD simulation data show good agreement with experimental results.
Graphic abstract












Similar content being viewed by others
References
Chung IM, Hemapriya V, Kim SH, Ponnusamy K, Arunadevi N, Chitra S, Prabakaran M, Gopiraman M (2021) Liriope platyphylla extract as a green inhibitor for mild steel corrosion in sulfuric acid medium. Chem Eng Commun 208(1):72–88
Elkholy AE, El-TaibHeakal F (2018) Electrochemical measurements and semi-empirical calculations for understanding adsorption of novel cationic Gemini surfactant on carbon steel in H2SO4 solution. J Mol Struct 1156:473–482
Barrahi M, Elhartiti H, El Mostaphi A, Chahboun N, Saadouni M, Salghi R, Zarrouk A, Ouhssine M (2019) Corrosion inhibition of mild steel by Fennel seeds (Foeniculum vulgare Mill) essential oil in 1 M hydrochloric acid solution. Int J Corros Scale Inhib 8(4):937–953
Abdel Nazeer A, Madkour M (2018) Potential use of smart coatings for corrosion protection of metals and alloys. J Mol Liq 253:11–22
Heusler KE, Landolt D, Trasatti S (1989) Electrochemical corrosion nomenclature. J Electroanal Chem Interfacial Electrochem 274:345–348
Soltani N, Tavakkoli N, Attaran A, Karimi B, Khayatkashani M (2020) Inhibitory effect of Pistacia khinjuk aerial part extract for carbon steel corrosion in sulfuric acid and hydrochloric acid solutions. Chem Pap 74:1799–1815
Chai C, Xu Y, Li D, Zhao X, Xu Y, Zhanga L, Wu Y (2019) Cysteamine modified polyaspartic acid as a new class of green corrosion inhibitor for mild steel in sulfuric acid medium: synthesis, electrochemical, surface study and theoretical calculation. Prog Org Coat 129:159–170
Laabaissi T, Benhiba F, Rouifi Z, Missioui M, Ourrak K, Oudda H, Ramli Y, Warad I, Allali M, Zarrouk A (2019) New quinoxaline derivative as a green corrosion inhibitor for mild steel in mild acidic medium: Electrochemical and theoretical studies. Int J Corros Scale Inhib 8(2):241–256
Nabah R, Benhiba F, Ramli Y, Ouakki M, Cherkaoui M, Oudda H, Touir R, Warad I, Zarrouk A (2018) Corrosion inhibition study of 5, 5-diphenylimidazolidine-2, 4-dione for mild steel corrosion in 1 M HCl solution: experimental, theoretical computational and Monte Carlo simulations studies. Anal Bioanal Electrochem 10(10):1375–1398
Ma X, Dang R, Kang Y, Gong Y, Luo J, Zhang Y, Fu J, Li C, Ma Y (2020) Electrochemical studies of expired drug (formoterol) as oilfield corrosion inhibitor for mild steel in H2SO4 media. Int J Electrochem Sci 15:1964–1981
Zarrok H, Zarrouk A, Salghi R, Ramli Y, Hammouti B, Assouag M, Essassi EM, Oudda H, Taleb M (2012) 3,7-Dimethylquinoxalin-2-(1H)-one for inhibition of acid corrosion of carbon steel. J Chem Pharm Res 4(12):5048–5055
Hamdani I, El Ouariachi E, Mokhtari O, Salhi A, Chahboun N, El Mahi B, Bouyanzer A, Zarrouk A, Hammouti B, Costa J (2015) Chemical constituents and corrosion inhibition of mild steel by the essential oil of thymus algeriensis in 1.0 M hydrochloric acid solution. Der Pharm Chem 7(8):252–264
El-Rabiei MM, Nady H, Zaki EG, Negem M (2019) Theoretical and experimental investigation of the synergistic infuence of tricine and iodide ions on the corrosion control of carbon steel in sulfuric acid electrolyte. J Bio Tribo Corros 5:103. https://doi.org/10.1007/s40735-019-0298-5
Rbaa M, Galai M, Abousalem AS, Lakhrissi B, EbnTouhami M, Warad I, Zarrouk A (2020) Synthetic, spectroscopic characterization, empirical and theoretical investigations on the corrosion inhibition characteristics of mild steel in molar hydrochloric acid by three novel 8-hydroxyquinoline derivatives. Ionics 26:503–522
Fergachi O, Benhiba F, Rbaa M, Ouakki M, Galai M, Touir R, Lakhrissi B, Oudda H, Ebn Touhami M (2019) Corrosion inhibition of ordinary steel in 5.0 M HCl medium by benzimidazole derivatives: electrochemical, UV–visible spectrometry, and DFT calculations. J Bio Tribo Corros 5:21. https://doi.org/10.1007/s40735-018-0215-3
El Faydy M, Rbaa M, Lakhrissi L, Lakhrissi B, Warad I, Zarrouk A, Obot IB (2019) Corrosion protection of carbon steel by two newly synthesized benzimidazol-2-ones substituted 8-hydroxyquinoline derivatives in 1 M HCl: experimental and theoretical study. Surf Interfaces 14:222–237
Guo L, Tan J, Kaya S, Leng S, Li Q, Zhang F (2020) Multidimensional insights into the corrosion inhibition of 3,3- dithiodipropionic acid on Q235 steel in H2SO4 medium: a combined experimental and in silico investigation. J Colloid Interface Sci 570:116–124
Zarrouk A, Hammouti B, Zarrok H, Salghi R, Dafali A, Bazzi Lh, Bammou L, Al-Deyab SS (2012) Electrochemical impedance spectroscopy and weight loss study for new pyridazine derivative as inhibitor for copper in nitric acid. Der Pharm Chem 4(1):337–346
Zarrok H, Al Mamari K, Zarrouk A, Salghi R, Hammouti B, Al-Deyab SS, Essassi EM, Bentiss F, Oudda H (2012) Gravimetric and electrochemical evaluation of 1-allyl-1Hindole-2,3-dione of carbon steel corrosion in hydrochloric acid. Int J Electrochem Sci 7:10338–10357
Louadi YE, Abrigach F, Bouyanzer A, Touzani R, El Assyry A, Zarrouk A, Hammouti B (2017) Theoretical and experimental studies on the corrosion inhibition potentials of two tetrakis pyrazole derivatives for mild steel in 10 M HCl. Port Electrochim Acta 35(3):159–178
Radhakrishnan S, Shimmon R, Conn C, Baker A (2015) Integrated kinetic studies and computational analysis on naphthyl chalcones as mushroom tyrosinase inhibitors. Bioorg Med Chem Lett 25(19):4085–4091
Xu X, Li J, Du C, Song Y (2011) Improved synthesis of 1,3-Diaryl-2-propen-1-one oxime in the presence of anhydrous sodium sulfate. Chin J Chem 29(12):2781–2784
Qian Y, Ma GY, Yang Y, Cheng K, Zheng QZ, Mao WJ, Shi L, Zhao J, Zhu HL (2010) Synthesis, molecular modeling and biological evaluation of dithiocarbamates as novel antitubulin agents. Bioorg Med Chem 18:4310–4316
Wang YT, Qina YJ, Zhang YL, LiY J, Rao B, Zhang YQ, Yang M, Jiang AQ, Qi JL, Zhu HL (2014) Synthesis, biological evaluation, and molecular docking studies of novel chalcone oxime derivatives as potential tubulin polymerization inhibitors. RSC Adv 4:32263–32275
Zarrok H, Zarrouk A, Salghi R, Oudda H, Hammouti B, Assouag M, Taleb M, EbnTouhami M, Bouachrine M, Boukhris S (2012) Gravimetricandquantumchemical studies of 1-[4-acetyl-2-(4- chlorophenyl)quinoxalin-1(4H)-yl]acetone as corrosion inhibitor for carbon steel in hydrochloric acid solution. J Chem Pharm Res 4(12):5056–5066
Tazouti A, Galai M, Touir R, EbnTouhami M, Zarrouk A, Ramli Y, Saraçoğlu M, Kaya S, Kandemirli F, Kaya C (2016) Experimental and theoretical studies for mild steel corrosion inhibition in 1.0 M HCl by three new quinoxalinone derivatives. J Mol Liq 221:815–832
Zarrouk A, Hammouti B, Dafali A, Zarrok H (2011) L-Cysteine methyl ester hydrochloride: a new corrosion inhibitor for copper in nitric acid. Der Pharma Chem 3(4):266–274
Echchihbi E, Nahlé A, Salim R, Benhiba F, Moussaif A, El-Hajjaji F, Oudda H, Guenbour A, Taleb M, Warad I, Zarrouk A (2020) Computational, MD simulation, SEM/EDX and experimental studies for understanding adsorption of benzimidazole derivatives as corrosion inhibitors in 10 M HCl solution. J Alloys Compd 844:155842
Accelrys (2016) Materials studio, revision 8.0. Accelrys Inc., San Diego
Andersen HC (1980) Molecular dynamics simulations at constant pressure and/or temperature. J Chem Phys 72:2384–2393
Benhiba F, Elaoufir Y, Belayachi M, Zarrok H, El Assyry A, Zarrouk A, Hammouti B, Ebenso EE, Guenbour A, Al Deyab SS, Oudda H (2014) Theoretical and experimental studies on the inhibition of 1,1’-(2-phenylquinoxaline 1,4-diyl)diethanone for the corrosion of carbon steel in 1.0 M HCl. Der Pharm Lett 6(4):306–318
Laamari MR, Benzakour J, Berrekhis F, Bakasse M, Villemin D (2012) Investigation of the effect of piperidin-1-yl-phosphonic acid on corrosion of iron in sulfuric acid. Arab J Chem 9(S2):S1218–S1224
Solmaz R, Mert ME, Kardaş G, Yazici B, Erbıl M (2008) Adsorption and corrosion inhibition effect of 1,1′-thiocarbonyldiimidazole on mild steel in H2SO4 solution and synergistic effect of iodide ion. Acta Phys Chim Sin 24:1185–1191
Derfouf H, Harek Y, Larabi L, Basirun WJ, Ladan M (2019) Corrosion inhibition activity of carbon steel in 1.0 M hydrochloric acid medium using hammada scoparia extract: gravimetric and electrochemical study. J Adhes Sci Technol 33(8):808–833
Li X, Xie X, Deng S, Du G (2014) Two phenylpyrimidine derivatives as new corrosion inhibitors for cold rolled steel in hydrochloric acid solution. Corros Sci 87:27–39
Khadraoui A, Khelifa A, Hadjmeliani M, Mehdaou R, Hachama K, Tidu A, Azari Z, Obot IB, Zarrouk A (2016) Extraction, characterization and anti-corrosion activity of Mentha pulegium oil: weight loss, electrochemical, thermodynamic and surface studies. J Mol Liq 216:724–731
Anusuya N, Saranya J, Sounthari P, Zarrouk A, Chitra S (2017) Corrosion inhibition and adsorption behaviour of some bis-pyrimidine derivatives on mild steel in acidic medium. J Mol Liq 225:406–417
Döner A, Solmaz R, Özcan M, Kardas G (2011) Experimental and theoretical studies of thiazoles as corrosion inhibitors for mild steel in sulphuric acid solution. Corros Sci 53:2902–2913
Bentiss F, Lebrini M, Vezin H, Chai F, Traisnel M, Lagrené M (2009) Enhanced corrosion resistance of carbon steel in normal sulfuric acid medium by some macrocyclic polyether compounds containing a 1,3,4-thiadiazole moiety: AC impedance and computational studies. Corros Sci 51:2165–2173
Boucherit L, Douadi T, Chafai N, Al-Noaimi M, Chafaa S (2018) The inhibition activity of 1,10–bis(2-formylphenyl)-1,4,7,10–tetraoxadecane (Ald) and its schiff base (L) on the corrosion of carbon steel in HCl: experimental and theoretical studies. Int J Electrochem Sci 13:3997–4025
Fiala A, Boukhedena W, Lemallem SE, Ladouani HB, Allal H (2019) Inhibition of carbon steel corrosion in HCl and H2SO4 solutions by ethyl 2-cyano-2-(1,3-dithian-2-ylidene) acetate. J Bio Tribo Corros 5:42. https://doi.org/10.1007/s40735-019-0237-5
Saranya J, Benhiba F, Anusuya N, Subbiah R, Zarrouk A, Chitra S (2020) Experimental and computational approaches on the pyran derivatives for acid corrosion. Colloids Surf A 603:125231
El Ouadi Y, Abrigach F, Bouyanzer A, Touzani R, Riant O, ElMahi B, El Assyry A, Radi S, Zarrouk A, Hammouti B (2015) Corrosion inhibition of mild steel by new N-heterocyclic compound in 1 M HCl: experimental and computational study. Der Pharma Chem 7(8):265–275
Mashuga ME, Olasunkanmi LO, Verma C, Sherif ESM, Ebenso EE (2020) Experimental and computational mediated illustration of effect of different substituents on adsorption tendency of phthalazinone derivatives on mild steel surface in acidic medium. J Mol Liq 305:112844
Boutouil A, Laamari MR, Elazhary I, Bahsis L, Anane H, Stiriba SE (2020) Towards a deeper understanding of the inhibition mechanism of a new 1,2,3-triazole derivative for mild steel corrosion in the hydrochloric acid solution using coupled experimental and theoretical methods. Mater Chem Phys 241:122420
El Faydy M, Galai M, EbnTouhami M, Obot IB, Lakhrissi B, Zarrouk A (2017) Anticorrosion potential of some 5-amino-8-hydroxyquinolines derivatives on carbon steel in hydrochloric acid solution: gravimetric, electrochemical, surface morphological, UV–visible, DFT and Monte Carlo simulations. J Mol Liq 248:1014–1027
Khattabi M, Benhiba F, Tabti S, Djedouani A, El Assyry A, Touzani R, Warad I, Oudda H, Zarrouk A (2019) Performance and computational studies of two soluble pyran derivatives as corrosion inhibitors for mild steel in HCl. J Mol Struct 1196:231–244
Shaban SM (2016) N-(3-(dimethyl benzyl ammonio) propyl) alkanamide chloride derivatives as corrosion inhibitors for mild steel in 1M HCl solution: experimental and theoretical investigation. RSC Adv 6:39784–39800
Shaban SM, Aiad I, El-Sukkary MM, Soliman EA, El-Awady MY (2015) Inhibition of mild steel corrosion in acidic medium by vanillin cationic surfactants. J Mol Liq 203:20–28
Kumar R, Yadav OS, Singh G (2017) Electrochemical and surface characterization of a new ecofriendly corrosion inhibitor for mild steel in acidic media: a cumulative study. J Mol Liq 237:413–427
Díaz-Cardenas MY, Valladares-Cisneros MG, Lagunas-Rivera S, Salinas-Bravo VM, Lopez-Sesenes R, Gonzalez-Rodríguez JG (2017) Peumusboldus extract as corrosion inhibitor for carbon steel in 0.5 M sulfuric acid. Green Chem Lett Rev 10(4):257–268
Zarrouk A, Hammouti B, Al-Deyab SS, Salghi R, Zarrok H, Jama C, Bentiss F (2012) Corrosion inhibition performance of 3,5-diamino-1,2,4-triazole for protection of copper in nitric acid solution. Int J Electrochem Sci 7:5997–6601
Zarrok H, Zarrouk A, Salghi R, Oudda H, Hammouti B, Ebn Touhami M, Bouachrine M, Boukhris S (2012) A combined experimental and theoretical study on the corrosion inhibition and adsorption behaviour of quinoxaline derivative during carbon steel corrosion in hydrochloric acid. Port Electrochim Acta 30(6):405–417
Rajendraprasad S, Ali S, Prasanna BM (2020) Electrochemical behavior of N1-(3-methylphenyl)piperidine-1,4-dicarboxamide as a corrosion inhibitor for soft-cast steel carbon steel in 1 M HCl. J Fail Anal Preven 20:235–241
Zarrok H, Salghi R, Zarrouk A, Hammouti B, Oudda H, Bazzi Lh, Bammou L, Al-Deyab SS (2012) Investigation of the inhibition effect of N-1-naphthylethylenediamine dihydrochloride monomethanolate on the C38 steel corrosion in 0.5M H2SO4. Der Pharma Chem 4(1):407–416
Singh A, Ansari KR, Kumar A, Liu W, Songsong C, Lin Y (2017) Electrochemical, surface and quantum chemical studies of novel imidazole derivatives as corrosion inhibitors for J55 steel in sweet corrosive environment. J Alloys Compd 712:121–133
Kharbach Y, Qachchachi FZ, Haoudi A, Tourabi M, Zarrouk A, Jama C, Olasunkanmi LO, Ebenso EE, Bentiss F (2017) Anticorrosion performance of three newly synthesized isatin derivatives on carbon steel in hydrochloric acid pickling environment: electrochemical, surface and theoretical studies. J Mol Liq 246:302–316
Salhi A, Tighadouini S, El-Massaoudi M, Elbelghiti M, Bouyanzer A, Radi S, El Barkany S, Bentiss F, Zarrouk A (2017) Keto-enol heterocycles as new compounds of corrosion inhibitors for carbon steel in 1 M HCl: weight loss, electrochemical and quantum chemical investigation. J Mol Liq 248:340–349
Solomon MM, Umoren SA (2016) In-situ preparation, characterization and anticorrosion property of polypropylene glycol/silver nanoparticles composite for mild steel corrosion in acid solution. J Colloid Interface Sci 462:29–41
Prabakarana M, Kima SH, Oha YT, Rajb V, Chung IM (2017) Anticorrosion properties of momilactone a isolated from rice hulls. J Ind Eng Chem 45:380–386
Saxena A, Prasad D, Haldhar R (2018) Use of Asparagus racemosus extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J Mater Sci 53:8523–8535
Rbaa M, Fardioui M, Verma C, Abousalem AS, Galai M, Ebenso EE, Guedira T, Lakhrissi B, Warad I, Zarrouk A (2020) 8-Hydroxyquinoline based chitosan derived carbohydrate polymer as biodegradable and sustainable acid corrosion inhibitor for mild steel: experimental and computational analyses. Int J Biol Macromol 155:645–655
Cao S, Liu D, Zhang P, Yang L, Yang P, Lu H, Gui J (2017) Green Brönsted acid ionic liquids as novel corrosion inhibitors for carbon steel in acidic medium. Sci Rep 7:8773. https://doi.org/10.1038/s41598-017-07925-y
El Yaktini A, Lachiri A, El Faydy M, Benhiba F, Zarrok H, El Azzouzi M, Zertoubi M, Azzi M, Lakhrissi B, Zarrouk A (2018) Practical and theoretical study on the inhibitory inflences of new azomethine derivatives containing 8-hydroxyquinoline moiety for the corrosion of carbon steel in 1 M HCl. Orient J Chem 34(6):3016–3029
Cherrak K, Benhiba F, Sebbar NK, Essassi EM, Taleb M, Zarrouk A, Dafali A (2019) Corrosion inhibition of mild steel by new benzothiazine derivative in a hydrochloric acid solution: experimental evaluation and theoretical calculations. Chem Data Collect 22:100252
Rouifi Z, Benhiba F, El Faydy M, Laabaissi T, About H, Oudda H, Warad I, Guenbour A, Lakhrissi B, Zarrouk A (2019) Performance and computational studies of new soluble triazole as corrosion inhibitor for carbon steel in HCl. Chem Data Collect 22:100242
Benhiba F, Benzekri Z, Guenbour A, Tabyaoui M, Bellaouchou A, Boukhris S, Oudda H, Warad I, Zarrouk A (2020) Combined electronic/atomic level computational, surface (SEM/EDS), chemical and electrochemical studies of the mild steel surface by quinoxalines derivatives anti-corrosion properties in 1 mol. L-1 HCl solution. Chin J Chem Eng 28(5):1436–1458
Saha SK, Ghosh P, Hens A, Murmu NC, Banerjee P (2015) Density functional theory and molecular dynamics simulation study on corrosion inhibition performance of mild steel by mercapto-quinoline Schiff base corrosion inhibitor. Phys E 66:332–341
Rbaa M, Benhiba F, Dohare P, Lakhrissi L, Touir R, Lakhrissi B, Zarrouk A, Lakhrissi Y (2020) Synthesis of new epoxy glucose derivatives as a non-toxic corrosion inhibitors for carbon steel in molar HCl: experimental, DFT and MD simulation. Chem Data Collect 27:100394
Rahmani H, Alaoui KI, El Azzouzi M, Benhiba F, El Hallaoui A, Rais Z, Taleb M, Saady A, Labriti B, Aouniti A, Zarrouk A (2019) Corrosion assessement of mild steel in acid environment using novel triazole derivative as a anti-corrosion agent: a combined experimental and quantum chemical study. Chem Data Collect 24:100302
Olasunkanmi LO, Obot IB, Ebenso EE (2016) Adsorption and corrosion inhibition properties of N-{n-[1-R-5-(quinoxalin-6-yl)-4, 5-dihydropyrazol-3-yl] phenyl} methanesulfonamides on mild steel in 1 M HCl: experimental and theoretical studies. RSC Adv 6:86782–86797
Benhiba F, Hsissou R, Benzekri Z, Belghiti ME, Lamhamdi A, Bellaouchou A, Guenbour A, Boukhris S, Oudda H, Warad I, Zarrouk A (2020) Nitro substituent effect on the electronic behavior and inhibitory performance of two quinoxaline derivatives in relation to the corrosion of mild steel in 1 M HCl. J Mol Liq 312:113367
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thoume, A., Benhiba, F., Elmakssoudi, A. et al. Corrosion inhibition behavior of chalcone oxime derivatives on carbon steel in 0.5 M H2SO4. J Appl Electrochem 51, 1755–1770 (2021). https://doi.org/10.1007/s10800-021-01612-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-021-01612-7