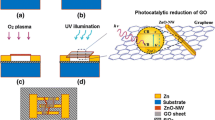
Abstract
The heterostructure of graphene and ZnO nanorods is attractive as a tin oxide-free electron transport layer for a broad variety of excitonic photovoltaic technologies. This work focuses on the effect of electrodeposition variables on morphology and performance of vertically aligned zinc oxide nanorods (ZVNRs) on graphene. This in situ growth technique has potential for fabrication of a wide variety of graphene heterostructures under mild synthesis conditions to prevent graphene damage. Large area graphene was grown by chemical vapor deposition, stacked up to four atomic layers, and transferred to glass. ZVNRs were electrodeposited on the graphene-coated glass and the topography was controlled by changing the electrodeposition parameters of the time, temperature, stirring, and seeding layers. The mechanisms controlling the cathodic electrodeposition of nanocrystals on graphene were studied by scanning electron microscopy of the ZVNRs topography. The effect of the topography of the ZVNRs on the electron generation and transport was studied for photoanode application in reference dye-sensitized solar cells. The charge transfer resistance and kinetics of the materials as photoanodes were measured with the techniques of linear sweep voltammetry, open circuit voltage decay, and electrochemical impedance spectroscopy. The optimization of ZnO growth resulted in an increase of the surface-to-volume ratio of the electrode from 10 to 250 mm−1, 60-fold increase of electron lifetime and ten-fold increase in power output. The results of this study provide fundamental understanding for designing electrodeposition processes of the hybrid ZVNR/graphene material.
Graphic abstract








Similar content being viewed by others
Data availability
Data and materials used in the study are available upon request to the email cchaves@itcr.ac.cr.
References
Mathew S, Yella A, Gao P, Humphry-Baker R, Curchod BFE, Ashari-Astani N, Tavernelli I, Rothlisberger U, Nazeeruddin MK, Gratzel M (2014) Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat Chem 6:242–247. https://doi.org/10.1038/nchem.1861
Li T, Lee Y, Teng H (2012) High-performance quantum dot-sensitized solar cells based on sensitization with CuInS2 quantum dots/CdS heterostructure. Energy Environ Sci 5:5315–5324. https://doi.org/10.1039/C1EE02253A
Chandrasekhar PS, Komarala VK (2015) Effect of graphene and Au@SiO2 core-shell nano-composite on photoelectrochemical performance of dye-sensitized solar cells based on N-doped titania nanotubes. Rsc Adv 5:84423–84431. https://doi.org/10.1039/C5RA13799C
Kazim S, Nazeeruddin MK, Graetzel M, Ahmad S (2014) Perovskite as light harvester: a game changer in photovoltaics. Angew Chem Int Ed 53:2812–2824. https://doi.org/10.1002/anie.201308719
Thakur UK, Askar AM, Kisslinger R, Wiltshire BD, Kar P, Shankar K (2017) Halide perovskite solar cells using monocrystalline TiO2 nanorod arrays as electron transport layers: impact of nanorod morphology. Nanotechnology 28:274001. https://doi.org/10.1088/1361-6528/aa75ab
Thavasi V, Lazarova T, Filipek S, Kolinski M, Querol E, Kumar A, Ramakrishna S, Padros E, Renugopalakrishnan V (2009) Study on the feasibility of bacteriorhodopsin as bio-photosensitizer in excitonic solar cell: a first report. J Nanosci Nanotechnol 9:1679–1687. https://doi.org/10.1166/jnn.2009.si07
Mershin A, Matsumoto K, Kaiser L, Yu D, Vaughn M, Nazeeruddin MK, Bruce BD, Graetzel M, Zhang S (2012) Self-assembled photosystem-I biophotovoltaics on nanostructured TiO2 and ZnO. Sci Rep 2:234. https://doi.org/10.1038/srep00234
Badding MA, Fix NR, Orandle MS, Barger MW, Dunnick KM, Cummings KJ, Leonard SS (2016) Pulmonary toxicity of indium-tin oxide production facility particles in rats. J Appl Toxicol 36:618–626. https://doi.org/10.1002/jat.3253
Polgreen L (2008) Congo’s riches, looted by renegade troops. The New York Times. https://www.nytimes.com/2008/11/16/world/africa/16congo.html. Accessed 12 Aug 2015
Commission S A E (2012) Final Rule-CONFLICT MINERALS. Securities and Exchange Commission. https://www.sec.gov/rules/final/2012/34-67716.pdf S.A.E. Accessed 12 Aug 2015
Xu XZ, Zhang ZH, Dong JC, Yi D, Niu JJ, Wu MH, Lin L, Yin RK, Li MQ, Zhou JY, Wang SX, Sun JL, Duan XJ, Gao P, Jiang Y, Wu XS, Peng HL, Ruoff RS, Liu ZF, Yu DP, Wang EG, Ding F, Liu KH (2017) Ultrafast epitaxial growth of metre-sized single-crystal graphene on industrial Cu foil. Sci Bull 62:1074–1080. https://doi.org/10.1016/j.scib.2017.07.005
Zhang QF, Dandeneau CS, Zhou XY, Cao GZ (2009) ZnO nanostructures for dye-sensitized solar cells. Adv Mater 21:4087–4108. https://doi.org/10.1002/adma.200803827
Kayes BM, Atwater HA, Lewis NS (2005) Comparison of the device physics principles of planar and radial p-n junction nanorod solar cells. J Appl Phys 97:114302. https://doi.org/10.1063/1.1901835
Lewis NS (2007) Toward cost-effective solar energy use. Science 315:798–801. https://doi.org/10.1126/science.1137014
Mu QH, Li YG, Zhang QH, Wang HZ (2011) Template-free formation of vertically oriented TiO2 nanorods with uniform distribution for organics-sensing application. J Hazard Mater 188:363–368. https://doi.org/10.1016/j.jhazmat.2011.01.125
Chandrasekhar PS, Dubey A, Qiao Q (2020) High efficiency perovskite solar cells using nitrogen-doped graphene/ZnO nanorod composite as an electron transport layer. Sol Energy 197:78–83. https://doi.org/10.1016/j.solener.2019.12.062
Mamaghani KR, Naghib SM (2017) The effect of stirring rate on electrodeposition of nanocrystalline nickel coatings and their corrosion behaviors and mechanical characteristics. Int J Electrochem Sci 12:5023–5035. https://doi.org/10.20964/2017.06.68
Izaki M, Omi T (1996) Transparent zinc oxide films prepared by electrochemical reaction. Appl Phys Lett 68:2439–2440. https://doi.org/10.1063/1.116160
Khajavi MR, Blackwood DJ, Cabanero G, Tena-Zaera R (2012) New insight into growth mechanism of ZnO nanowires electrodeposited from nitrate-based solutions. Electrochim Acta 69:181–189. https://doi.org/10.1016/j.electacta.2012.02.096
Elias J, Tena-Zaera R, Levy-Clement C (2007) Electrodeposition of ZnO nanowires with controlled dimensions for photovoltaic applications: role of buffer layer. Thin Solid Films 515:8553–8557. https://doi.org/10.1016/j.tsf.2007.04.027
Rosas-Laverde NM, Pruna A, Busquets-Mataix D, Pullini D (2020) Graphene oxide-assisted morphology and structure of electrodeposited ZnO nanostructures. Materials 13:365. https://doi.org/10.3390/ma13020365
Xu C, Lee JH, Lee JC, Kim BS, Hwang SW, Whang D (2011) Electrochemical growth of vertically aligned ZnO nanorod arrays on oxidized bi-layer graphene electrode. CrystEngComm 13:6036–6039. https://doi.org/10.1039/C1CE05695F
Hambali NA, Yahaya H, Mahmood MR, Terasako T, Hashim AM (2014) Synthesis of zinc oxide nanostructures on graphene/glass substrate by electrochemical deposition: effects of current density and temperature. Nanoscale Res Lett 9:609. https://doi.org/10.1186/1556-276X-9-609
Huang S, Wu PF, Yue HY et al (2019) ZnO nanosheet arrays/graphene foam: voltammetric determination of dopamine in the presence of ascorbic acid and uric acid. J Mater Sci 30:16510–16517. https://doi.org/10.1007/s10854-019-02027-z
Gao LB, Ren WC, Xu HL, Jin L, Wang ZX, Ma T, Ma LP, Zhang ZY, Fu Q, Peng LM, Bao XH, Cheng HM (2012) Repeated growth and bubbling transfer of graphene with millimetre-size single-crystal grains using platinum. Nat Commun 3:699. https://doi.org/10.1038/ncomms1702
Villarreal CC, Pirzada D, Wong A, Vi D, Pham T, Mulchandani A (2018) Characterisation of the heterojunction microstructure for electrodeposited vertical ZnO nanorods on CVD-graphene. Mater Res Express 5:085031. https://doi.org/10.1088/2053-1591/aace06
Malekpour H, Ramnani P, Srinivasan S, Balasubramanian G, Nika DL, Mulchandani A, Lake R, Balandin AA (2016) Thermal conductivity of graphene with defects induced by electron beam irraditiation. Nanoscale 8:14608–14616. https://doi.org/10.1039/C6NR03470E
Zarebska K, Kwiatkowski M, Gniadek M, Skompska M (2013) Electrodeposition of Zn(OH)(2), ZnO thin films and nanosheet-like Zn seed layers and influence of their morphology on the growth of ZnO nanorods. Electrochim Acta 98:255–262. https://doi.org/10.1016/j.electacta.2013.03.051
Wang MS, Jiang LX, Kim EJ, Hahn SH (2015) Electronic structure and optical properties of Zn(OH)(2): LDA+U calculations and intense yellow luminescence. RSC Adv 5:87496–87503. https://doi.org/10.1039/C5RA17024A
Zaban A, Greenshtein M, Bisquert J (2003) Determination of the electron lifetime in nanocrystalline dye solar cells by open-circuit voltage decay measurements. ChemPhysChem 4:859–864. https://doi.org/10.1002/cphc.200200615
Sarker S, Ahammad AJS, Seo HW, Kim DM (2014) Electrochemical impedance spectra of dye-sensitized solar cells: fundamentals and spreadsheet calculation. Int J Photoenergy 2014:851705. https://doi.org/10.1155/2014/851705
Bisquert J (2002) Theory of the impedance of electron diffusion and recombination in a thin layer. J Phys Chem B 106:325–333. https://doi.org/10.1021/jp011941g
Scharifker B, Hills G (1983) Theoretical and experimental studies of multiple nucleation. Electrochim Acta 28:879–889. https://doi.org/10.1016/0013-4686(83)85163-9
Son DY, Bae KH, Kim HS, Park NG (2015) Effects of seed layer on growth of ZnO nanorod and performance of perovskite solar cell. J Phys Chem C 119:10321–10328. https://doi.org/10.1021/acs.jpcc.5b03276
Wipperman K, Schultze JW, Kessel R, Penninger J (1991) The inhibition of zinc corrosion by bisaminotriazole and other triazole derivatives. Corros Sci 32:205–230. https://doi.org/10.1016/0010-938X(91)90044-P
Nasirpouri F (2017) Electrodeposition of nanostructured materials. Springer, Cham 62:1–325. https://doi.org/10.1007/978-3-319-44920-3
Goux A, Pauporte T, Chivot LD (2005) Temperature effects on ZnO electrodeposition. Electrochim Acta 50:2239–2248. https://doi.org/10.1016/j.electacta.2004.10.007
Dupuy L, Haller S, Rousset J, Donsanti F, Guillemoles FG, Lincot D, Decker (2010) Impedance measurements of nanoporosity in electrodeposited ZnO films for DSSC. Electrochem Commun 12:697–699. https://doi.org/10.1016/j.elecom.2010.03.009
Yin Z, Wu S, Zhou X, Huang X, Zhang Q, Boey F, Zhang H (2009) Electrochemical deposition of ZnO nanorods on transparent reduced graphene oxide electrodes for hybrid solar cells. Small 6:307–312. https://doi.org/10.1002/smll.200901968
Wan WT, Zhu LP, Hu L, Chen GF, Mi WB, Ye ZZ (2014) Investigation of morphology evolution of Cu-ZnO nanorod arrays and enhancement of ferromagnetism by codoping with N. Phys Lett A 378:2763–2767. https://doi.org/10.1016/j.physleta.2014.07.040
Tatiparti SSV, Ebrahimi F (2012) Potentiostatic versus galvanostatic electrodeposition of nanocrystalline Al-Mg alloy powders. J Solid State Electrochem 16:1255–1262. https://doi.org/10.1007/s10008-011-1522-5
Bera D, Qian L, Sabui S, Santra S, Holloway PH (2008) Photoluminescence of ZnO quantum dots produced by a sol-gel process. Opt Mater 30:1233–1239. https://doi.org/10.1016/j.optmat.2007.06.001
Greene LE, Law M, Goldberger J, Kim F, Johnson JC, Zhang YF, Saykally RJ, Yang PD (2003) Low-temperature wafer-scale production of ZnO nanowire arrays. Angew Chem Int Ed Engl 42:3031–3034. https://doi.org/10.1002/anie.200351461
Hsieh CT, Yang SY, Lin JY (2010) Electrochemical deposition and superhydrophobic behavior of ZnO nanorod arrays. Thin Solid Films 518:4884–4889. https://doi.org/10.1016/j.tsf.2010.03.081
Bhardwaj M, Balani K, Balasubramaniam R, Pandey S, Agarwal A (2011) Effect of current density and grain refining agents on pulsed electrodeposition of nanocrystalline nickel. Surf Eng 27(9):642–648. https://doi.org/10.1179/026708410x12683118611185
Salazar R, Lévy-Clément C, Ivanova V (2012) Galvanostatic deposition of ZnO thin films. Electrochim Acta 78:547–556. https://doi.org/10.1016/j.electacta.2012.06.070
Acknowledgements
A.M. acknowledges the financial support from W. Ruel Johnson Chair in Environmental Engineering. C.V. acknowledges Instituto Tecnológico de Costa Rica for the scholarship to pursue her Ph.D. studies at UC Riverside.
Funding
The study was performed with the financial support from W. Ruel Johnson Chair in Environmental Engineering. Instituto Tecnológico de Costa Rica supported the Ph.D. studies of C.V. in UC Riverside with a scholarship.
Author information
Authors and Affiliations
Contributions
CV was responsible for device design, materials fabrication, electrochemical measurements and original draft preparation; DP and AW collaborated in materials fabrication and electrochemical measurements; AW revised the manuscript; AM guided the revision of the manuscript and design of the study. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest or competing interests regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Villarreal, C.C., Pirzada, D., Wong, A. et al. Electrodeposition of ZnO nanorods on graphene: tuning the topography for application as tin oxide-free electron transport layer. J Appl Electrochem 51, 977–989 (2021). https://doi.org/10.1007/s10800-021-01531-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-021-01531-7