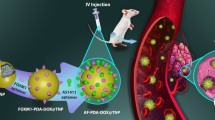
Abstract
Aptamers are affinity molecules with high specificity, proposed as excellent alternatives to antibodies in targeting and detecting applications due to their smaller size, higher stability, and simplicity of production and modification compared with antibodies. Due to lack of a sensitive and simple method to quantitatively evaluate attachment of aptamer to nanoparticles (NPs), optimization of the attachment process was not considered in most of previously studied aptamer-targeted drug delivery systems. The aim of current study was to demonstrate the utility of electrochemical impedance spectroscopy (EIS) technique in this field. Ecoflex® polymeric NPs loaded with docetaxel (DTX-NPs) were fabricated via electrospraying technique, and HER-2-specific aptamer molecules were attached via amide bonds (Apt-DTX-NPs). Using EIS method, the time period of various stages of aptamer conjugation was optimized, by comparing the amount of aptamer molecules attached to the DTX-NPs. The results of in vitro studies on optimum Apt-DTX-NPs demonstrated that the proposed delivery system could significantly enhance the cellular uptake and the cytotoxic effect against HER-2 positive cell line in comparison with non-targeted or Herceptin-targeted DTX-NPs. Thus, aptamer conjugation could improve the in vitro performance of Ecoflex NPs, which could be suggested as a potential DTX delivery system in HER-2 overexpressing cancers. In this regard, EIS method could play its role as a sensitive quantification method to obtain the optimized aptamer-conjugated NP systems.
Graphical abstract







Similar content being viewed by others
Abbreviations
- EIS:
-
Electrochemical impedance spectroscopy
- Apt:
-
Aptamer
- DTX:
-
Docetaxel
- ER:
-
Estrogen
- PR:
-
Progesterone
- EGFR:
-
Epidermal growth factor receptor
- AC:
-
Alternating current
- MTT:
-
3-[4, 5-Dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide
- EDC:
-
N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
- DMF:
-
N,N-dimethyl formamide
- NHS:
-
N-hydroxysuccinimide
- RPMI:
-
Roswell Park Memorial Institute
- FBS:
-
Fetal bovine serum
- NPs:
-
Nanoparticles
- DTX-NPs:
-
Non-targeted docetaxel nanoparticles
- Blank NPs:
-
Docetaxel-free nanoparticles
- Apt-NPs:
-
Docetaxel-free aptamer-targeted nanoparticles
- Hcp-NPs:
-
Docetaxel-free Herceptin-targeted nanoparticles
- Apt-DTX-NPs:
-
Aptamer-targeted docetaxel nanoparticles
- Hcp-DTX-NPs:
-
Herceptin-targeted docetaxel nanoparticles
- RhB:
-
Rhodamine B
References
Anderson WF, Matsuno R (2006) Breast cancer heterogeneity: a mixture of at least 2 main types. J Natl Cancer Inst 98:948–951
Basu S, Chen W, Tchou J, Mavi A, Cermik T, Czerniecki B et al (2008) Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters. Cancer 112:995–1000
Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V (2007) Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype. Cancer 109:1721–1728
Shen G, Yu H, Bian G, Gao M, Liu L, Cheng M et al (2013) Genistein inhibits the proliferation of human HER2-positive cancer cells by downregulating HER2 receptor. Functional Foods in Health Disease 3:291–299
O’Sullivan CK (2002) Aptasensors—the future of biosensing. Anal Bioanal Chem 372:44–48
Murphy MB, Fuller ST, Richardson PM, Doyle SA (2003) An improved method for the in vitro evolution of aptamers and applications in protein detection and purification. Nucleic Acids Res 31:110
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822
Peyrin E (2009) Nucleic acid aptamer molecular recognition principles and application in liquid chromatography and capillary electrophoresis. J Sep Sci 32:1531–1536
Wang RE, Wu H, Niu Y, Cai J (2011) Improving the stability of aptamers by chemical modification. Curr Med Chem 18:4126–4138
Mascini M, Palchetti I, Tombelli S (2012) Nucleic acid and peptide aptamers: fundamentals and bioanalytical aspects. Angew Chem Int Ed Engl 51:1316–1332
Ates M (2011) Review study of electrochemical impedance spectroscopy and equivalent electrical circuits of conducting polymers on carbon surfaces. Prog Org Coat 71:1–10
Schwake A, Geuking H, Cammann K (1998) Application of a new graphical fitting approach for data analysis in electrochemical impedance spectroscopy. Electroanalysis 10:1026–1029
Gebala M, Schuhmann W (2010) Controlled orientation of DNA in a binary SAM as a key for the successful determination of DNA hybridization by means of electrochemical impedance spectroscopy. Chem Phy Chem 11:2887–2895
Shelley M, Harrison C, Coles B, Staffurth J, Wilt TJ, Mason MD (2006) Chemotherapy for hormone-refractory prostate cancer. Cochrane Database Syst Rev 4:1–70
van Poppel H (2005) Recent docetaxel studies establish a new standard of care in hormone refractory prostate cancer. Can J Urol 12:81–85
Baker J, Ajani J, Scotte F, Winther D, Martin M, Aapro MS et al (2009) Docetaxel-related side effects and their management. Eur J Oncol Nurs 13:49–59
Perez EA (2009) Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol Cancer Ther 8:2086–2095
Youm I, Yang XY, Murowchick JB, Youan BC (2011) Encapsulation of docetaxel in oily core polyester nanocapsules intended for breast cancer therapy. Nanoscale Res Lett 6:30
Mahapatro A, Singh DK Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnology 9: 55–66
Varshosaz J, Riahi S, Ghassami E, Jahanian-Najafabadi A (2017) Transferrin-targeted poly(butylene adipate)/terephthalate nanoparticles for targeted delivery of 5-fluorouracil in HT29 colorectal cancer cell line. J Bioact Compat Pol 32:503–527
Kim MY, Jeong S (2011) In vitro Selection of RNA aptamer and specific targeting of ErbB2 in breast cancer cells. Nucleic Acid Ther 21:73–178
Tabasi A, Noorbakhsh A, Sharifi E (2017) Reduced graphene oxide-chitosan-aptamer interface as new platform for ultrasensitive detection of human epidermal growth factor receptor 2. Biosens Bioelectron 95:117–123
Varshosaz J, Ghassami E, Noorbakhsh A, Jahanian-Najafabadi A, Minayian M, Behzadi R (2018) Poly (butylene adipate-co-butylene terephthalate) nanoparticles prepared by electrospraying technique for docetaxel delivery in ovarian cancer induced mice. Drug Dev Ind Pharm 44:1012–1022
Ghassami E, Varshosaz J, Jahanian-Najafabadi A, Minaiyan M, Rajabi P, Hayati E (2018) Pharmacokinetics and in vitro/in vivo antitumor efficacy of aptamer-targeted Ecoflex® nanoparticles for docetaxel delivery in ovarian cancer. Int J Nanomed 13:493–504
Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW et al (2006) Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. PNAS 103:6315–6320
Farokhzad OC, Jon S, Khademhosseini A, Tran TNT, LaVan DA, Langer R (2004) Nanoparticle-aptamer bioconjugates: a new approach for targeting prostate cancer cells. Cancer Res 64:7668–7672
Varshosaz J, Taymouri S, Hassanzadeh F, Haghjooy Javanmard S, Rostami M (2015) Folated synperonic-cholesteryl hemisuccinate polymeric micelles for the targeted delivery of docetaxel in melanoma. Biomed Res Int 1–17
Anido J, Matar P, Albanell J, Guzma´n M, Rojo F, Arribas J et al (2003) ZD1839 a specific epidermal growth factor receptor (egfr) tyrosine kinase inhibitor induces the formation of inactive egfr/her2 and egfr/her3 heterodimers and prevents heregulin signaling in her2-overexpressing breast cancer cells. Clin Cancer Res 9:1274–1283
Xu Z, Chen L, Gu W, Gao Y, Lin L, Zhang Z et al (2009) The performance of docetaxel-loaded solid lipid nanoparticles targeted to hepatocellular carcinoma. Biomaterials 30:226–232
Hu Z, Tan J, Lai Z, Zheng R, Zhong J, Wang Y et al (2017) aptamer combined with fluorescent silica nanoparticles for detection of hepatoma cells. Nanoscale Res Lett 12:96
Teymourian H, Salimi A, Firoozia S (2013) A high performance electrochemical biosensing platform for glucose detection and ige aptasensing based on Fe3O4/reduced graphene oxide nanocomposite. Electroanalysis 25:1–10
Chen Z, Li L, Zhao H, Guo L, Mu X (2011) Electrochemical impedance spectroscopy detection of lysozyme based on electrodeposited gold nanoparticles. Talanta 83:1501–1506
Zhang WJ (2016) Application of Fe3O4 nanoparticles functionalized carbon nanotubes for electrochemical sensing of DNA hybridization. Appl Electrochem 46:559
Min K, Song KM, Cho M, Chun YS, Shim YB et al (2010) Simultaneous electrochemical detection of both PSMA (+) and PSMA(-) prostate cancer cells using an RNA/peptide dual-aptamer probe. Chem Commun 46:5566–5568
Yu C, Hu Y, Duan J, Yuan W, Wang C, Xu H et al (2011) Novel aptamer-nanoparticle bioconjugates enhances delivery of anticancer drug to MUC1-positive cancer cells in vitro. PLOS One 6:1–8
Sanna V, Roggio AM, Posadino AM, Cossu A, Marceddu S, Mariani A et al (2011) Novel docetaxel-loaded nanoparticles based on poly(lactide-co-caprolactone) and poly(lactideco-glycolide-co-caprolactone) for prostate cancer treatment: formulation, characterization, and cytotoxicity studies. Nanoscale Res Lett 6:260
Acknowledgements
The authors appreciate financial support of Isfahan University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Varshosaz, J., Ghassami, E., Noorbakhsh, A. et al. Implementation of electrochemical impedance spectroscopy to evaluate HER-2 aptamer conjugation to Ecoflex® nanoparticles for docetaxel delivery in breast cancer cells. J Appl Electrochem 49, 87–97 (2019). https://doi.org/10.1007/s10800-018-1273-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-018-1273-4