Abstract
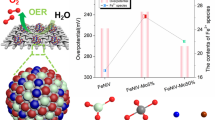
Electrochemical catalysts for the oxygen evolution reaction (OER) play a key role in highly-efficient water splitting and many other important energy conversion applications. Transition metal oxides are promising OER catalysts. In this work, Fe, W co-doped Co3O4 was grown on carbon fiber cloth (FeWCo3O4/CFC) and polypyrrole (PPy)/carbon fiber cloth (FeWCo3O4/PPy/CFC) through a simple anodic electrodeposition method. The FeWCo3O4/CFC free-standing electrode reached an electrocatalytic current density of 30.7 mA cm−2 at 400 mV overpotential with a Tafel slope of 177 mV dec−1. The PPy can serve as conductive binder and improve the contact between FeWCo3O4 and substrate. The resulting FeWCo3O4/PPy/CFC free-standing electrode reached an electrocatalytic current density of 36.2 mA cm−2 at 400 mV overpotential with a Tafel slope of 163 mV dec−1. The FeWCo3O4/PPy/CFC free-standing electrode shows low electric resistance and is able to catalyze OER at 10 mA cm−2 for 12 h without obvious decay under the optimized electrodeposition conditions. This study provides new insight for design and synthesis of highly-efficient OER catalyst.
Graphical Abstract






Similar content being viewed by others
References
Chu S, Majumdar A (2012) Opportunities and challenges for a sustainable energy future. Nature 488(7411):294–303
Turner JA (2004) Sustainable Hydrogen production. Science 305(5686):972–974. https://doi.org/10.1126/science.1103197
Walter MG, Warren EL, McKone JR, Boettcher SW, Mi Q, Santori EA, Lewis NS (2010) Solar water splitting cells. Chem Rev 110(11):6446–6473
Dresselhaus M, Thomas I (2001) Alternative energy technologies. Nature 414(6861):332–337
Zou X, Zhang Y (2015) Noble metal-free hydrogen evolution catalysts for water splitting. Chem Soc Rev 44(15):5148–5180. https://doi.org/10.1039/c4cs00448e
Pletcher D, Li X (2011) Prospects for alkaline zero gap water electrolysers for hydrogen production. Int J Hydrogen Energy 36(23):15089–15104
Reier T, Oezaslan M, Strasser P (2012) Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials. ACS Catal 2(8):1765–1772
Ma W, Ma R, Wang C, Liang J, Liu X, Zhou K, Sasaki T (2015) A superlattice of alternately stacked Ni–Fe hydroxide nanosheets and graphene for efficient splitting of water. ACS Nano 9(2):1977–1984
Zhang B, Zheng X, Voznyy O, Comin R, Bajdich M, García-Melchor M, Han L, Xu J, Liu M, Zheng L, García de Arquer FP, Dinh CT, Fan F, Yuan M, Yassitepe E, Chen N, Regier T, Liu P, Li Y, De Luna P, Janmohamed A, Xin HL, Yang H, Vojvodic A, Sargent EH (2016) Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 352(6283):333–337. https://doi.org/10.1126/science.aaf1525
Lu Z, Wang H, Kong D, Yan K, Hsu PC, Zheng G, Yao H, Liang Z, Sun X, Cui Y (2014) Electrochemical tuning of layered lithium transition metal oxides for improvement of oxygen evolution reaction. Nat Commun 5:4345. https://doi.org/10.1038/ncomms5345
Maiyalagan T, Jarvis KA, Therese S, Ferreira PJ, Manthiram A (2014) Spinel-type lithium cobalt oxide as a bifunctional electrocatalyst for the oxygen evolution and oxygen reduction reactions. Nat Commun 5:3949. https://doi.org/10.1038/ncomms4949
Liu Y-C, Koza JA, Switzer JA (2014) Conversion of electrodeposited Co(OH)2 to CoOOH and Co3O4, and comparison of their catalytic activity for the oxygen evolution reaction. Electrochim Acta 140:359–365. https://doi.org/10.1016/j.electacta.2014.04.036
Zhou Y, Lee CW, Kim SK, Yoon S (2012) Ordered mesoporous carbon/MoO2 nanocomposites as stable supercapacitor electrodes. ECS Electrochem Lett 1(1):A17–A20. https://doi.org/10.1149/2.013201eel
Gong M, Dai H (2014) A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Research 8(1):23–39. https://doi.org/10.1007/s12274-014-0591-z
Seh ZW, Kibsgaard J, Dickens CF, Chorkendorff I, Norskov JK, Jaramillo TF (2017) Combining theory and experiment in electrocatalysis: insights into materials design. Science 355(6321):eaad4998. https://doi.org/10.1126/science.aad4998
Huang J, Chen J, Yao T, He J, Jiang S, Sun Z, Liu Q, Cheng W, Hu F, Jiang Y, Pan Z, Wei S (2015) CoOOH nanosheets with high mass activity for water oxidation. Angew Chem Int Ed Engl 54(30):8722–8727. https://doi.org/10.1002/anie.201502836
Wang A-L, Xu H, Li G-R (2016) NiCoFe layered triple hydroxides with porous structures as high-performance electrocatalysts for overall water splitting. ACS Energy Lett 1(2):445–453. https://doi.org/10.1021/acsenergylett.6b00219
Jiang N, You B, Sheng M, Sun Y (2015) Electrodeposited cobalt-phosphorous-derived films as competent bifunctional catalysts for overall water splitting. Angew Chem Int Ed Engl 54(21):6251–6254. https://doi.org/10.1002/anie.201501616
Jiang N, You B, Sheng M, Sun Y (2016) Bifunctionality and mechanism of electrodeposited nickel-phosphorous films for efficient overall water splitting. ChemCatChem 8(1):106–112. https://doi.org/10.1002/cctc.201501150
Zhao S, Wang Y, Dong J, He C-T, Yin H, An P, Zhao K, Zhang X, Gao C, Zhang L, Lv J, Wang J, Zhang J, Khattak AM, Khan NA, Wei Z, Zhang J, Liu S, Zhao H, Tang Z (2016) Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nature Energy 1:16184. https://doi.org/10.1038/nenergy.2016.184
Zhuang Z, Sheng W, Yan Y (2014) Synthesis of monodispere Au@Co3O4 core-shell nanocrystals and their enhanced catalytic activity for oxygen evolution reaction. Adv Mater 26(23):3950–3955. https://doi.org/10.1002/adma.201400336
Zhao Y, Chen S, Sun B, Su D, Huang X, Liu H, Yan Y, Sun K, Wang G (2015) Graphene-Co(3)O(4) nanocomposite as electrocatalyst with high performance for oxygen evolution reaction. Sci Rep 5:7629. https://doi.org/10.1038/srep07629
Han X, Yu C, Zhou S, Zhao C, Huang H, Yang J, Liu Z, Zhao J, Qiu J (2017) Ultrasensitive iron-triggered nanosized Fe-CoOOH integrated with graphene for highly efficient oxygen evolution. Adv Energy Mater. https://doi.org/10.1002/aenm.201602148
Shang C, Li M, Wang Z, Wu S, Lu Z (2016) Electrospun nitrogen-doped carbon nanofibers encapsulating cobalt nanoparticles as efficient oxygen reduction reaction catalysts. ChemElectroChem 3(9):1437–1445. https://doi.org/10.1002/celc.201600275
Liu Y, Liu Y, Cheng SH-S, Yu S, Nan B, Bian H, Md K, Wang M, Chung CY, Lu Z-G (2016) Conformal coating of heterogeneous CoO/Co nanocomposites on carbon nanotubes as efficient bifunctional electrocatalyst for Li-air batteries. Electrochim Acta 219:560–567. https://doi.org/10.1016/j.electacta.2016.10.064
Unni SM, Dhavale VM, Pillai VK, Kurungot S (2010) High Pt utilization electrodes for polymer electrolyte membrane fuel cells by dispersing Pt particles formed by a preprecipitation method on carbon “polished” with polypyrrole. J Phys Chem C 114(34):14654–14661. https://doi.org/10.1021/jp104664t
Bashyam R, Zelenay P (2006) A class of non-precious metal composite catalysts for fuel cells. Nature 443(7107):63–66. https://doi.org/10.1038/nature05118
Olson TS, Pylypenko S, Atanassov P, Asazawa K, Yamada K, Tanaka H (2010) Anion-exchange membrane fuel cells: dual-site mechanism of oxygen reduction reaction in alkaline media on cobalt–polypyrrole electrocatalysts. J Phys Chem C 114(11):5049–5059. https://doi.org/10.1021/jp910572g
Diallo A, Beye AC, Doyle TB, Park E, Maaza M (2015) Green synthesis of Co3O4 nanoparticles via Aspalathus linearis: physical properties. Green Chem Lett Rev 8(3–4):30–36. https://doi.org/10.1080/17518253.2015.1082646
Wang HY, Hung SF, Chen HY, Chan TS, Chen HM, Liu B (2016) In operando identification of geometrical-site-dependent water oxidation activity of spinel Co3O4. J Am Chem Soc 138(1):36–39. https://doi.org/10.1021/jacs.5b10525
Acknowledgements
This work was supported by the Hong Kong Polytechnic University (Project No. RUKQ).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, Q., Liu, Y., Ma, L. et al. PPy enhanced Fe, W Co-doped Co3O4 free-standing electrode for highly-efficient oxygen evolution reaction. J Appl Electrochem 48, 1189–1195 (2018). https://doi.org/10.1007/s10800-018-1211-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-018-1211-5