Abstract
Spinel LiMn2O4-based cathode material has been successfully commercialized for power lithium ion batteries for large-scale applications in pure electric vehicles. However, pure LiMn2O4 suffers from poor rate performance and fast capacity fading especially at elevated temperatures derived from Mn dissolution and structural distortion. Herein, a study on the rate and cycle performance of single/double-cation doped porous LiMn2O4 microspheres, which was prepared by an easy method using porous MnCO3 microspheres as a self-supporting template, was performed. The as-synthesized porous Li1.02Co0.05Mn1.90Li0.05O4 (LMO-S4) microspheres constructed with nanometer-sized primary particles show an obvious enhancement of cyclability over other LiMn2O4-based materials such as Li1.02Mn2O4 (LMO-S1), Li1.02Mn1.95Li0.05O4 (LMO-S2) and Li1.02Co0.05Mn1.95O4 (LMO-S3), especially at an elevated temperature (55 °C). The obtained LMO-S4/lithium half cells deliver capacities of 113.1 and 109.0 mAh g−1 at 1.0 and 5 C, respectively, with the corresponding capacity retentions of 88.9 and 90.2% for up to 1000 cycles. Meanwhile, it can deliver an initial capacity of 114.0 mAh g−1 at 5 C with a capacity retention of 80.1% after 1000 cycles at 55 °C. Furthermore, it displays superior rate performance and cycle performance at 0 °C with a specific capacity of 106 mAh g−1, and the capacity retention is 79.6% after 1000 cycles at 5 C. These results reveal that a dual-doping strategy and porous structure design play synergistic roles in the preparation of high performance LiMn2O4-based spinel cathode material. The cation co-doped strategy can maintain the crystal structural stability and provide interfacial stability while preserving fast Li+ diffusion during the long-time cycling at elevated temperatures. Furthermore, the porous structure favors fast Li+ intercalation/deintercalation kinetics by allowing electrolyte insertion through the nanoparticles during the reversible electrochemical process.
Graphical Abstract
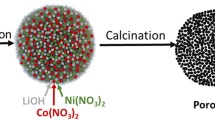
Lithium and cobalt co-doped LiMn2O4 with a nominal composition of Li1.02Co0.05Mn1.90Li0.05O4 exhibits an obviously improved cycle performance at high temperature than that of single-doped LiMn2O4.












Similar content being viewed by others
References
Tarascon JM, Armand M (2001) Determination of the chemical diffusion coefficient of lithium in LiFePO4. Nature 414:359–367
Wu FX, Yushin G (2017) Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energy Environ Sci 10:435–459
Deng YF, Yang CX, Zou KX, Qin XS, Zhao ZX, Chen GH (2017) Recent advances of Mn-rich LiFe1–yMnyPO4 (0.5 ≤ y < 1.0) cathode materials for high energy density lithium ion batteries. Adv Energy Mater 7:1601958
Jin Y, Zhu B, Lu ZD, Liu N, Zhu J (2017) Challenges and recent progress in the development of Si anodes for lithium-ion battery. Adv Energy Mater 7:1700715
Li WD, Song BH, Manthiram A (2017) High-voltage positive electrode materials for lithium-ion batteries. Chem Soc Rev 46:3006–3059
Liu W, Kowal K, Farrington GC (1998) Mechanism of the electrochemical insertion of lithium into LiMn2O4 spinels. J Electrochem Soc 145:459–465
Park OK, Cho Y, Lee S, Yoo HC, Song HK, Cho J (2011) Who will drive electric vehicles, olivine or spinel? Energy Environ Sci 4:1621–1633
Xu GJ, Liu ZH, Zhang CJ, Cui GL, Chen LQ (2015) Strategies for improving the cyclability and thermo-stability of LiMn2O4-based batteries at elevated temperatures. J Mater Chem A 3:4092–4123
Thackeray MM, Shao-Horn Y, Kahaian AJ, Kepler KD, Vaughey JT, Hackney SA (1998) Structural fatigue in spinel electrodes in high voltage (4 V) Li/LixMn2O4 cells. Electrochem Solid State Lett 1:7–9
Chung KY, Ryu CW, Kim KB (2005) Onset mechanism of Jahn-Teller distortion in 4 V LiMn2O4 and its suppression by LiM0.05Mn1.95O4 (M = Co, Ni) coating. J Electrochem Soc 152:A791-A795
Dai Y, Cai L, White RE (2013) Capacity fade model for spinel LiMn2O4 electrode. J Electrochem Soc 160:A182-A190
Liu CF, Neale ZG, Cao GZ (2016) Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater Today 19:109–123
Jang DH, Shin YJ, Oh SM (1996) Dissolution of spinel oxides and capacity losses in 4 V Li/LixMn2O4 cells. J Electrochem Soc 143:2204–2211
Kim D, Park S, Chae OB, Ryu JH, Kim YU, Yin RZ, Oh SM (2012) Re-deposition of manganese species on spinel LiMn2O4 electrode after Mn dissolution. J Electrochem Soc 159:A193–A197
Hirayama M, Ido H, Kim K, Cho W, Tamura K, Mizuki JI, Kanno R (2010) Dynamic structural changes at LiMn2O4/electrolyte interface during lithium battery reaction. J Am Chem Soc 132:15268–15276
Zhan C, Lu J, Kropf AJ, Wu TP, Jansen AN, Sun YK, Qiu XP, Amine K (2013) Mn(II) deposition on anodes and its effects on capacity fade in spinel lithium manganite-carbon systems. Nature Commun 4:2437
Myung ST, Komaba S, Kumagai N (2001) Enhanced structural stability and cyclability of Al-doped LiMn2O4 spinel synthesized by the emulsion drying method. J Electrochem Soc 148:A482–A489
Sun YK, Yoon CS, Kim CK, Youn SG, Lee Y-S, Yoshio M, Oh I-H (2001) Degradation mechanism of spinel LiAl0.2Mn1.8O4 cathode materials on high temperature cycling. J Mater Chem 11:2519–2522
Shen CH, Liu RS, Gundakaram R, Chen JM, Huang SM, Chen JS, Wang SM (2001) Effect of Co doping in LiMn2O4. J Power Sources 102:21–28
Amatucci G, Tarascon J-M (2002) Optimization of insertion compounds such as LiMn2O4 for Li-ion batteries. J Electrochem Soc 149:K31–K46
Xia YG, Wang HY, Zhang Q, Nakamura H, Noguchi H, Yoshio M (2007) Oxygen deficiency, a key factor in controlling the cycle performance of Mn-spinel cathode for lithium-ion batteries. J Power Sources 166:485–491
Xiong LL, Xu YL, Tao T, Song J, Goodenough JB (2012) Excellent stability of spinel LiMn2O4-based composites for lithium ion batteries. J Mater Chem 22:24563–24568
Lee DK, Han SC, Ahn D, Singh SP, Sohn KS, Pyo M (2012) Suppression of phase transition in LiTb0. 01Mn1. 99O4 cathodes with fast Li+ diffusion. ACS Appl Mater Interfaces 4:6841–6847
Han DW, Ryu WH, Kim WK, Eom JY, Kwon HS (2013) Effects of Li and Cl codoping on the electrochemical performance and structural stability of LiMn2O4 cathode materials for hybrid electric vehicle applications. J Phys Chem C 117:4913–4919
Yu FD, Wang ZB, Chen F, Wu J, Zhang XG, Gu DM (2014) Crystal structure and multicomponent effects in Li1 + xMn2–x–yAlyO4 cathode materials for Li-ion batteries. J Power Sources 262:104–111
Wen WC, Ju BW, Wang XY, Wu C, Shu HB, Yang XK (2014) Effects of magnesium and fluorine co-doping on the structural and electrochemical performance of the spinel LiMn2O4 cathode materials. Electrochim Acta 147:271–278
Yang CX, Tan HQ, Deng YF, Qin XS, Li YW, Chen GH (2017) Importance of synergistic role of cobalt and aluminum on a greatly improved electrochemical performance of Li-rich oxyfluoride spinel at elevated-temperature. J Alloy Comp 728:612–622
Pasqualini M, Calcaterra S, Maroni F, Rezvani SJ, Di Cicco A, Alexander S, Rajantie H, Tossici R, Nobili F (2017) Electrochemical and spectroscopic characterization of an alumina-coated LiMn2O4 cathode with enhanced interfacial stability. Electrochim Acta 258:175–181
Lee M-J, Lee S, Oh P, Kim Y, Cho J (2014) High performance LiMn2O4 cathode materials grown with epitaxial layered nanostructure for Li-ion batteries. Nano Lett 14:993–999
Shang YS, Lin XJ, Lu X, Huang T, Yu AS (2015) Nano-TiO2(B) coated LiMn2O4 as cathode materials for lithium-ion batteries at elevated temperatures. Electrochim Acta 156:121–126
Jeong M, Lee MJ, Cho J, Lee S (2015) Surface Mn oxidation state controlled spinel LiMn2O4 as a cathode material for high-energy Li-ion batteries. Adv Energy Mater 5:1500440
Lee S, Yoon G, Jeong M, Lee MJ, Kang K, Cho J (2015) Hierarchical surface atomic structure of a manganese-based spinel cathode for lithium-ion batteries. Angew Chem Int Ed 54:1153–1158
Waller GH, Brooke PD, Rainwater BH, Lai SY, Hu R, Ding Y, Alamgir FM, Sandhage KH, Liu ML (2016) Structure and surface chemistry of Al2O3 coated LiMn2O4 nanostructured electrodes with improved lifetime. J Power Sources 306:162–170
Cheng FY, Wang HB, Zhu ZQ, Wang Y, Zhang TR, Tao ZL, Chen J (2011) Porous LiMn2O4 nanorods with durable high-rate capability for rechargeable Li-ion batteries. Energy Environ Sci 4:3668–3675
Deng YF, Zhou YB, Shi ZC, Zhou X, Quan X, Chen GH (2013) Porous LiMn2O4 microspheres as durable high power cathode materials for lithium ion batteries. J Mater Chem A 1:8170–8177
Kim JS, Kim K, Cho W, Shin WH, Kanno R, Choi JW (2012) A truncated manganese spinel cathode for excellent power and lifetime in lithium-ion batteries. Nano Lett 12:6358–6365
Fu Y, Jiang H, Hu YJ, Dai YH, Zhang L, Li CZ (2015) Synergistic enhancement effect of Al doping and highly active facets of LiMn2O4 cathode materials for lithium-ion batteries. Ind Eng Chem Res 54:3800–3805
Huang SS, Wu H, Chen PH, Guo Y, Nie B, Chen BJ, Liu H, Zhang Y (2015) Facile pH-mediated synthesis of morphology-tunable MnCO3 and their transformation to truncated octahedral spinel LiMn2O4 cathode materials for superior lithium storage. J Mater Chem A 3:3633–3640
Jiang CH, Tang ZL, Wang ST, Zhang ZT (2017) A truncated octahedral spinel LiMn2O4 as high-performance cathode material for ultrafast and long-life lithium-ion batteries. J Power Sources 357:144–148
Wen W, Chen S, Fu Y, Wang X, Shu H (2015) A core-shell structure spinel cathode material with a concentration-gradient shell for high performance lithium-ion batteries. J Power Sources 274:219–228
Yang CX, Deng YF, Gao M, Yang XF, Qin XS, Chen GH (2017) High-rate and long-life performance of a truncated spinel cathode material with off-stoichiometric composition at elevated temperature. Electrochim Acta 225:198–206
Li B, Wang YQ, Rong HB, Wang YT, Liu JS, Xing LD, Xu MQ, Li WS (2013) A novel electrolyte with the ability to form a solid electrolyte interface on the anode and cathode of a LiMn2O4/graphite battery. J Mater Chem A 1:12954–12961
Yamagiwa K, Morita D, Yabuuchi N, Tanaka T, Fukunishi M, Taki T, Watanabe H, Otsuka T, Yano T, Son JY (2015) Improved high-temperature performance and surface chemistry of graphite/LiMn2O4 Li-ion cells by fluorosilane-based electrolyte additive. Electrochim Acta 160:347–356
Zhang Z, Zen T, Lai YQ, Ji M, Li J (2014) A comparative study of different binders and their effects on electrochemical properties of LiMn2O4 cathode in lithium ion batteries. J Power Sources 247:1–8
Chiu K-F, Su SH, Leu H-J, Chen YS (2014) Application of lithiated perfluorosulfonate ionomer binders to enhance high rate capability in LiMn2O4 cathodes for lithium ion batteries. Electrochim Acta 117:134–138
Ryou M-H, Lee YM, Park J-K, Choi JW (2011) Mussel-inspired polydopamine-treated polyethylene separators for high-power Li-ion batteries. Adv Mater 23:3066–3071
Chen JJ, Wang SQ, Cai DD, Wang HH (2014) Porous SiO2 as a separator to improve the electrochemical performance of spinel LiMn2O4 cathode. J Membr Sci 449:169–175
Liu JT, Li G, Bai HL, Shao MM, Su CW, Guo JM, Liu SF, Bai W (2017) Enhanced cycle and rate performances of Li(Li0.05Al0.05Mn1.90)O4 cathode material prepared via a solution combustion method for lithium-ion batteries. Solid State Ionics 307:79–89
Zhao HY, Li F, Liu XQ, Cheng C, Zhang Z, Wu Y, Xiong WQ, Chen B (2015) Effects of equimolar Mg (II) and Si (IV) co-doping on the electrochemical properties of spinel LiMn2–2xMgxSixO4 prepared by citric acid assisted sol-gel method. Electrochim Acta 151:263–269
Lee YS, Kumada N, Yoshio M (2001) Synthesis and characterization of lithium aluminum-doped spinel (LiAlxMn2–xO4) for lithium secondary battery. J Power Sources 96:376–384
Zhao HY, Liu SS, Wang ZW, Cai Y, Tan M, Liu XQ (2016) Enhanced elevated-temperature performance of LiAlxSi0.05Mg0.05Mn1.90−xO4 (0≤x≤0.08) cathode materials for high-performance lithium-ion batteries. Electrochim Acta 199:18–26
Acknowledgements
This work was supported by the Guangzhou Scientific and Technological Planning Project (201704030061), the NSFC/RGC Joint Research Scheme (Grant No. 21661162002 and N_HKUST601/16) and the linked project of Natural Science Foundation of China (U1407124) and Qinghai Province.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deng, Y., Wang, S., Zhou, Y. et al. The enhancement of rate and cycle performance of LiMn2O4 at elevated temperatures by the synergistic roles of porous structure and dual-cation doping. J Appl Electrochem 48, 1083–1094 (2018). https://doi.org/10.1007/s10800-018-1200-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-018-1200-8