Abstract
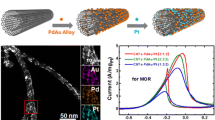
Electrocatalysts with high performance, increased stability, and high surface area are essential for the widespread commercialization of fuel cells. Nanostructured materials emerge as potential candidates to meet these targets. We report on the synthesis and characterization of a nanostructured platinum electrocatalyst for methanol oxidation. Using a simple, one-step electroless method, an interconnected platinum nanotube network was prepared. The highly ordered, high-surface-area catalyst exhibited enhanced current density and durability for methanol oxidation. The catalyst shows a peak current density of 1.43 mA cm−2 at 50 mV s−1 and retained 25% of its initial activity after 1 h. The high catalytic activity is a result of increased Pt–OH formation on nanocrystal aggregates at lower potentials.
Graphical Abstract








Similar content being viewed by others
References
Dresselhaus MS, Thomas IL (2001) Alternative energy technologies. Nature 414:332–337
Kamarudin SK, Achmad F, Daud WRW (2009) Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int J Hydrogen Energy 34:6902–6916
Arico AS, Srinivasan S, Antonucci V (2001) DMFCs: from fundamental aspects to technology development. Fuel Cells 1:133–161
Subhramannia M, Pillai VK (2008) Shape-dependent electrocatalytic activity of platinum nanostructures. J Mater Chem 18:5858–5870
Leger JM (2001) Mechanistic aspects of methanol oxidation on platinum-based electrocatalysts. J Appl Electrochem 31:767–771
Liu HS, Song CJ, Zhang L, Zhang JJ, Wang HJ, Wilkinson DP (2006) A review of anode catalysis in the direct methanol fuel cell. J Power Sources 155:95–110
Stamenkovic VR, Mun BS, Arenz M, Mayrhofer KJJ, Lucas C, Wang G, Ross PN, Markovic NM (2007) Trends in electrocatalysis on extended and nanoscale pt-bimetallic alloy surfaces. Nat Mater 6:241–247
Tripkovic AV, Popovic KD, Grgur BN, Blizanac B, Ross PN, Markovic NM (2002) Methanol electrooxidation on supported Pt and PtRu catalysts in acid and alkaline solutions. Electrochim Acta 47:3707–3714
Rhen FMF, McKeown C (2017) Enhanced methanol oxidation on strained Pt films. J Phys Chem C 121:2556–2562
Guo YG, Hu JS, Wan LJ (2008) Nanostructured materials for electrochemical energy conversion and storage devices. Adv Mater 20:2878–2887
Chen A, Holt-Hindle P (2010) Platinum-based nanostructured materials: synthesis, properties, and applications. Chem Rev 110:3767–3804
Narayanan R, El-Sayed MA (2004) Changing catalytic activity during colloidal platinum nanocatalysis due to shape changes: electron-transfer reaction. J Am Chem Soc 126:7194–7195
Lu B-A, Du J-H, Sheng T, Tian N, Xiao J, Liu L, Xu B-B, Zhou Z-Y, Sun S-G (2016) Hydrogen adsorption-mediated synthesis of concave Pt nanocubes and their enhanced electrocatalytic activity. Nanoscale 8:11559–11564
Zhou Z-Y, Huang Z-Z, Chen D-J, Wang Q, Tian N, Sun S-G (2010) High-index faceted platinum nanocrystals supported on carbon black as highly efficient catalysts for ethanol electrooxidation. Angew Chem Int Ed 49:411–414
Zhou L-N, Zhang X-T, Wang Z-H, Guo S, Li Y-J (2016) Cubic superstructures composed of PtPd alloy nanocubes and their enhanced electrocatalysis for methanol oxidation. Chem Commun 52:12737–12740
Peng Z, You H, Wu J, Yang H (2010) Electrochemical synthesis and catalytic property of Sub-10 nm platinum cubic nanoboxes. Nano Lett 10:1492–1496
Burda C, Chen X, Narayanan R, El-Sayed MA (2005) Chemistry and properties of nanocrystals of different shapes. Chem Rev 105:1025–1102
Koper MTM (2011) Structure sensitivity and nanoscale effects in electrocatalysis. Nanoscale 3:2054–2073
Xia BY, Wu HB, Yan Y, Lou XW, Wang X (2013) Ultrathin and ultralong single-crystal platinum nanowire assemblies with highly stable electrocatalytic activity. J Am Chem Soc 135:9480–9485
Lee H-B-R, Baeck SH, Jaramillo TF, Bent SF (2013) Growth of Pt nanowires by atomic layer deposition on highly ordered pyrolytic graphite. Nano Lett 13:457–463
Yin H, Zhao S, Zhao K, Muqsit A, Tang H, Chang L, Zhao H, Gao Y, Tang Z (2015) Ultrathin platinum nanowires grown on single-layered nickel hydroxide with high hydrogen evolution activity. Nat Commun 6:6430
Kawasaki JK, Arnold CB (2011) Synthesis of platinum dendrites and nanowires via directed electrochemical nanowire assembly. Nano Lett 11:781–785
Zhang G, Sun S, Cai M, Zhang Y, Li R, Sun X (2013) Porous dendritic platinum nanotubes with extremely high activity and stability for oxygen reduction reaction. Sci Rep 3:1526–1534
Khi NT, Yoon J, Baik H, Lee S, Ahn DJ, Kwon SJ, Lee K (2014) Twinning boundary-elongated hierarchical Pt dendrites with an axially twinned nanorod core for excellent catalytic activity. CrystEngComm 16:8312–8316
Sun YG, Mayers B, Xia YN (2003) Metal nanostructures with hollow interiors. Adv Mater 15:641–646
Bi Y, Lu G (2009) Control growth of uniform platinum nanotubes and their catalytic properties for methanol electrooxidation. Electrochem Commun 11:45–49
Ci S, Zou J, Zeng G, Luo S, Wen Z (2012) Single crystalline Pt nanotubes with superior electrocatalytic stability. J Mater Chem 22:16732–16737
Kim SM, Liu L, Kim DJ, Park S (2015) Vertically aligned double-walled platinum nanotubes decorated with inner fibrils for their enhanced electrocatalytic properties. J Phys Chem C 119:23075–23081
Alia SM, Zhang G, Kisailus D, Li DS, Gu S, Jensen K, Yan YS (2010) Porous platinum nanotubes for oxygen reduction and methanol oxidation reactions. Adv Funct Mater 20:3742–3746
Luo Y, Lee SK, Hofmeister H, Steinhart M, Gosele U (2004) Pt nanoshell tubes by template wetting. Nano Lett 4:143–147
Roscher V, Licklederer M, Schumacher J, Rios GR, Hoffmann B, Christiansen S, Bachmann J (2014) Accurate tuning of ordered nanotubular platinum electrodes by galvanic plating. Dalton Trans 43:4345–4350
Zhao Y, Guo YG, Zhang YL, Jiao K (2004) Fabrication and characterization of highly ordered Pt nanotubule arrays. Phys Chem Chem Phys 6:1766–1768
Muench F, Kaserer S, Kunz U, Svoboda I, Broetz J, Lauterbach S, Kleebe H-J, Roth C, Ensinger W (2011) Electroless synthesis of platinum and platinum-ruthenium nanotubes and their application in methanol oxidation. J Mater Chem 21:6286–6291
Chakarvarti SK, Vetter J (1998) Template synthesis—a membrane based technology for generation of nano-/micro materials: a review. Radiat Meas 29:149–159
Richardson D, Kingston S, Rhen FMF (2016) Investigation of the magnetization reversal mechanism of electrolessly deposited Co-B nanotubes. AIP Adv 6:056113
Rhen FMF, Richardson D, Pomar CAD, Souza JA (2016) Investigation of magnetic properties of Ni-B nanotubes at low temperatures. IEEE Trans Magn 52:1–4
Richardson D, Rhen FMF (2015) Increasing the magnetization of electrolessly deposited Ni-B nanotubes. IEEE Trans Magn 51:1–4
Richardson D, Rhen FMF (2015) Investigation of the electroless deposition process of magnetic nanostructures. ECS Trans 64:39–48
Brenner A, Riddell GE (1946) Nickel plating on steel by chemical reduction. J Res Natl Bur Stand 37:31–34
Ohno I (1991) Electrochemistry of electroless plating. Mater Sci Eng A 146:33–49
Shacham-Diamand Y, Osaka T, Okinaka Y, Sugiyama A, Dubin V (2015) 30 years of electroless plating for semiconductor and polymer micro-systems. Microelectron Eng 132:35–45
Wei X, Roper DK (2014) Tin sensitization for electroless plating review. J Electrochem Soc 161:D235–D242
Rao CRK, Trivedi DC (2005) Chemical and electrochemical depositions of platinum group metals and their applications. Coord Chem Rev 249:613–631
Koslov AS, Palanisamy T, Narasimhan D (2002) US Patent 6,391,477
Xia Y, Xiong Y, Lim B, Skrabalak SE (2009) Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew Chem Int Ed 48:60–103
Wulff G (1901) Zur Frage der Geschwindigkeit des Wachsthums und der Auflösung der Krystallflächen. Z Kristallogr Cryst Mater 34:449–530
Marks LD (1994) Experimental studies of small particle structures. Rep Prog Phys 57:603–649
Trasatti S, Petrii OA (1991) Real surface area measurements in electrochemistry. Pure Appl Chem 63:711–734
Scanlon MD, Salaj-Kosla U, Belochapkine S, MacAodha D, Leech D, Ding Y, Magner E (2012) Characterization of nanoporous gold electrodes for bioelectrochemical applications. Langmuir 28:2251–2261
Pajkossy T, Kolb DM (2001) Double layer capacitance of Pt(111) single crystal electrodes. Electrochim Acta 46:3063–3071
Tsou Y-M, Cao L, De Castro E (2008) Crucial role of low coordination sites in oxygen reduction, Co stripping, and size effect for nano-sized Pt particles. ECS Trans 13:67–84
Huang Y-F, Kooyman PJ, Koper MTM (2016) Intermediate stages of electrochemical oxidation of single-crystalline platinum revealed by in situ Raman Spectroscopy. Nat Commun 7:12440–12441
Brett CMA, Brett AMO (1993) Electrochemistry: principles, methods, and applications. Oxford University Press, Oxford, p 203
Mostafa E, Baltruschat H (2014) Quasi-continuous determination of the apparent transfer coefficient of methanol oxidation using a potential modulation technique under convection conditions. Electrocatal 5:75–86
Nordlund J, Lindbergh G (2004) Temperature-dependant kinetics of the anode in the DMFC. J Electrochem Soc 151:A1357–A1362
Nordlund J, Lindbergh G (2002) A model for the porous direct methanol fuel cells anode. J Electrochem Soc 149:A1107–A1113
Zhao X, Zhu J, Liang L, Liao J, Liu C, Xing W (2012) Enhanced activity of Pt nano-crystals supported on a novel TiO2@N-doped C nano-composite for methanol oxidation reaction. J Mater Chem 22:19718–19725
Wang X, Sun Y, Hu J, Li Y-J, Yeung ES (2015) Electrochemical fabrication of hydrangea macrophylla flower-like Pt hierarchical nanostructures and their properties for methanol electrooxidation. RSC Adv 5:13538–13543
Liu J, Chen B, Ni Z, Deng Y, Han X, Hu W, Zhong C (2016) Improving the electrocatalytic activity of Pt monolayer catalysts for electrooxidation of methanol, ethanol and ammonia by tailoring the surface morphology of the supporting core. ChemElectroChem 3:537–551
Zhang Q, Jiang F, Yue R, Du Y (2014) Electrochemically fabricated flower-like graphene as a highly efficient Pt electrocatalyst support for methanol oxidation. RSC Adv 4:12105–12108
Zhou LN, Zhang XT, Shen WJ, Sun SG, Li YJ (2015) Monolayer of close-packed pt nanocrystals on a reduced graphene oxide (RGO) nanosheet and its enhanced catalytic performance towards methanol electrooxidation. RSC Adv 5:46017–46025
Zhao X, Zhu J, Liang L, Li C, Liu C, Liao J, Xing W (2014) biomass-derived N-doped carbon and its application in electrocatalysis. Appl Catal B 154:177–182
Acknowledgements
This research is supported by Science Foundation Ireland grant number 12/IP/1692 and the HEA PRTLI4 programme (INSPIRE). The authors are thankful to Professor Edmond Magner, Professor Noel Buckley, and their teams for access to characterization facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McKeown, C., Rhen, F.M.F. Pt nanotube network with high activity for methanol oxidation. J Appl Electrochem 48, 165–173 (2018). https://doi.org/10.1007/s10800-017-1141-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-017-1141-7